Neurocognitive performance in family-based and case-control studies of schizophrenia
- PMID: 25432636
- PMCID: PMC4441547
- DOI: 10.1016/j.schres.2014.10.049
Neurocognitive performance in family-based and case-control studies of schizophrenia
Abstract
Background: Neurocognitive deficits in schizophrenia (SZ) are established and the Consortium on the Genetics of Schizophrenia (COGS) investigated such measures as endophenotypes in family-based (COGS-1) and case-control (COGS-2) studies. By requiring family participation, family-based sampling may result in samples that vary demographically and perform better on neurocognitive measures.
Methods: The Penn computerized neurocognitive battery (CNB) evaluates accuracy and speed of performance for several domains and was administered across sites in COGS-1 and COGS-2. Most tests were included in both studies. COGS-1 included 328 patients with SZ and 497 healthy comparison subjects (HCS) and COGS-2 included 1195 patients and 1009 HCS.
Results: Demographically, COGS-1 participants were younger, more educated, with more educated parents and higher estimated IQ compared to COGS-2 participants. After controlling for demographics, the two samples produced very similar performance profiles compared to their respective controls. As expected, performance was better and with smaller effect sizes compared to controls in COGS-1 relative to COGS-2. Better performance was most pronounced for spatial processing while emotion identification had large effect sizes for both accuracy and speed in both samples. Performance was positively correlated with functioning and negatively with negative and positive symptoms in both samples, but correlations were attenuated in COGS-2, especially with positive symptoms.
Conclusions: Patients ascertained through family-based design have more favorable demographics and better performance on some neurocognitive domains. Thus, studies that use case-control ascertainment may tap into populations with more severe forms of illness that are exposed to less favorable factors compared to those ascertained with family-based designs.
Conflict of interest statement
All other authors declare that they have no conflict of interest.
Figures
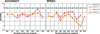
References
-
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Iowa City, Iowa: University of Iowa; 1984a.
-
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, Iowa: University of Iowa; 1984b.
-
- Braff DL. The importance of endophenotypes in schizophrenia research: Past, present and future. Schizophr. Res. this issue. - PubMed
Publication types
MeSH terms
Grants and funding
- MH065588/MH/NIMH NIH HHS/United States
- MH065554/MH/NIMH NIH HHS/United States
- MH86135/MH/NIMH NIH HHS/United States
- R01 MH065571/MH/NIMH NIH HHS/United States
- R01 MH065558/MH/NIMH NIH HHS/United States
- R01 MH065562/MH/NIMH NIH HHS/United States
- R01 MH042191/MH/NIMH NIH HHS/United States
- MH065571/MH/NIMH NIH HHS/United States
- R01 MH065707/MH/NIMH NIH HHS/United States
- MH065558/MH/NIMH NIH HHS/United States
- R01 MH093383/MH/NIMH NIH HHS/United States
- R01 MH086135/MH/NIMH NIH HHS/United States
- MH065578/MH/NIMH NIH HHS/United States
- MH065707/MH/NIMH NIH HHS/United States
- R01 MH065554/MH/NIMH NIH HHS/United States
- R01 MH065578/MH/NIMH NIH HHS/United States
- MH065562/MH/NIMH NIH HHS/United States
- R01 MH065588/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

