Photoreleasable ligands to study intracrine angiotensin II signalling
- PMID: 25433071
- PMCID: PMC4324703
- DOI: 10.1113/jphysiol.2014.279109
Photoreleasable ligands to study intracrine angiotensin II signalling
Abstract
Key points: The renin-angiotensin system plays a key role in cardiovascular physiology and its overactivation has been implicated in the pathogenesis of several major cardiovascular diseases. There is growing evidence that angiotensin II (Ang-II) may function as an intracellular peptide to activate intracellular/nuclear receptors and their downstream signalling effectors independently of cell surface receptors. Current methods used to study intracrine Ang-II signalling are limited to indirect approaches because of a lack of selective intracellularly-acting probes. Here, we present novel photoreleasable Ang-II analogues used to probe intracellular actions with spatial and temporal precision. The photorelease of intracellular Ang-II causes nuclear and cytosolic calcium mobilization and initiates the de novo synthesis of RNA in cardiac cells, demonstrating the application of the method.
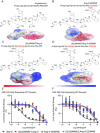
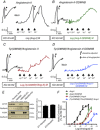
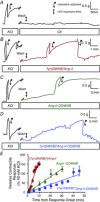
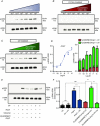
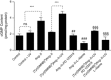
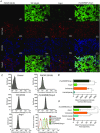
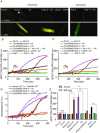
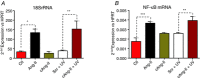
Abstract: Several lines of evidence suggest that intracellular angiotensin II (Ang-II) contributes to the regulation of cardiac contractility, renal salt reabsorption, vascular tone and metabolism; however, work on intracrine Ang-II signalling has been limited to indirect approaches because of a lack of selective intracellularly-acting probes. Here, we aimed to synthesize and characterize cell-permeant Ang-II analogues that are inactive without uncaging, but release active Ang-II upon exposure to a flash of UV-light, and act as novel tools for use in the study of intracrine Ang-II physiology. We prepared three novel caged Ang-II analogues, [Tyr(DMNB)(4)]Ang-II, Ang-II-ODMNB and [Tyr(DMNB)(4)]Ang-II-ODMNB, based upon the incorporation of the photolabile moiety 4,5-dimethoxy-2-nitrobenzyl (DMNB). Compared to Ang-II, the caged Ang-II analogues showed 2-3 orders of magnitude reduced affinity toward both angiotensin type-1 (AT1R) and type-2 (AT2R) receptors in competition binding assays, and greatly-reduced potency in contraction assays of rat thoracic aorta. After receiving UV-irradiation, all three caged Ang-II analogues released Ang-II and potently induced the contraction of rat thoracic aorta. [Tyr(DMNB)(4)]Ang-II showed the most rapid photolysis upon UV-irradiation and was the focus of subsequent characterization. Whereas Ang-II and photolysed [Tyr(DMNB)(4)]Ang-II increased ERK1/2 phosphorylation (via AT1R) and cGMP production (AT2R), caged [Tyr(DMNB)(4)]Ang-II did not. Cellular uptake of [Tyr(DMNB)(4)]Ang-II was 4-fold greater than that of Ang-II and significantly greater than uptake driven by the positive-control HIV TAT(48-60) peptide. Intracellular photolysis of [Tyr(DMNB)(4)]Ang-II induced an increase in nucleoplasmic Ca(2+) ([Ca(2+)]n), and initiated 18S rRNA and nuclear factor kappa B mRNA synthesis in adult cardiac cells. We conclude that caged Ang-II analogues represent powerful new tools for use in the selective study of intracrine signalling via Ang-II.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures

References
-
- Ando H, Furuta T, Tsien RY. Okamoto H. Photo-mediated gene activation using caged RNA/DNA in zebrafish embryos. Nat Genet. 2001;28:317–325. - PubMed
-
- Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG. Widdop RE. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci (Lond) 2011;121:297–303. - PubMed
-
- Bourgault S, Letourneau M. Fournier A. Development and pharmacological characterization of "caged" urotensin II analogs. Peptides. 2005;26:1475–1480. - PubMed
-
- Bourgault S, Letourneau M. Fournier A. Development of photolabile caged analogs of endothelin-1. Peptides. 2007;28:1074–1082. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

