Myh11(R247C/R247C) mutations increase thoracic aorta vulnerability to intramural damage despite a general biomechanical adaptivity
- PMID: 25433566
- PMCID: PMC4283495
- DOI: 10.1016/j.jbiomech.2014.10.031
Myh11(R247C/R247C) mutations increase thoracic aorta vulnerability to intramural damage despite a general biomechanical adaptivity
Abstract
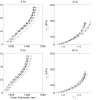
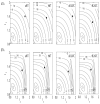
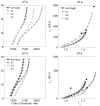
Genetic studies in patients reveal that mutations to genes that encode contractile proteins in medial smooth muscle cells can cause thoracic aortic aneurysms and dissections. Mouse models of such mutations, including Acta2(-/-) and Myh11(R247C/R247C), surprisingly do not present with any severe vascular phenotype under normal conditions. This observation raises the question whether these mutations nevertheless render the thoracic aorta increasingly vulnerable to aneurysms or dissections in the presence of additional, epigenetic, factors such as hypertension, a known risk factor for thoracic aortic disease. Accordingly, we compared the structure and biaxial mechanical properties of the ascending and descending thoracic aorta from male wild-type and Myh11(R247C/R247C) mice under normotension and induced hypertension. On average, the mutant aortas exhibited near normal biomechanics under normotensive hemodynamics and near normal adaptations to hypertensive hemodynamics, yet the latter led to intramural delaminations or premature deaths in over 20% of these mice. Moreover, the delaminated vessels exhibited localized pools of mucoid material, similar to the common histopathologic characteristic observed in aortas from humans affected by thoracic aortic aneurysms and dissections. The present findings suggest, therefore, that mutations to smooth muscle cell contractile proteins may place the thoracic aorta at increased risk to epigenetic factors and that there is a need to focus on focal, not global, changes in aortic structure and properties, including the pooling of glycosaminoglycans/proteoglycans that may lead to thoracic aortic dissection.
Keywords: Actomyosin functionality; Familial thoracic aortic aneurysms and dissections; Smooth muscle myosin heavy chain; Wall stress and stiffness.
Published by Elsevier Ltd.
Figures



References
-
- Chan KK, Rabkin SW. Increasing prevalence of hypertension among patients with thoracic aorta dissection: Trends over eight decades-a structured meta-analysis. Am J Hypertens. 2014 Jul;27(7):907–17. - PubMed
-
- Chen J, Wu J, Li L, Zou Y-Z, Zhu D-L, Gao P-J. Effect of an acute mechanical stimulus on aortic structure in the transverse aortic constriction mouse model. Clin Exp Pharmacol Physiol. 2011 Sep;38(9):570–6. - PubMed
-
- de Wit A, Vis K, Jeremy RW. Aortic stiffness in heritable aortopathies: relationship to aneurysm growth rate. Heart Lung Circ. 2013 Jan;22(1):3–11. - PubMed
-
- El-Hamamsy I, Yacoub MH. Cellular and molecular mechanisms of thoracic aortic aneurysms. Nat Rev Cardiol. 2009 Dec;6(12):771–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

