MFAP5 loss-of-function mutations underscore the involvement of matrix alteration in the pathogenesis of familial thoracic aortic aneurysms and dissections
- PMID: 25434006
- PMCID: PMC4259978
- DOI: 10.1016/j.ajhg.2014.10.018
MFAP5 loss-of-function mutations underscore the involvement of matrix alteration in the pathogenesis of familial thoracic aortic aneurysms and dissections
Abstract
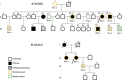
Thoracic aortic aneurysm and dissection (TAAD) is an autosomal-dominant disorder with major life-threatening complications. The disease displays great genetic heterogeneity with some forms allelic to Marfan and Loeys-Dietz syndrome, and an important number of cases still remain unexplained at the molecular level. Through whole-exome sequencing of affected members in a large TAAD-affected family, we identified the c.472C>T (p.Arg158(∗)) nonsense mutation in MFAP5 encoding the extracellular matrix component MAGP-2. This protein interacts with elastin fibers and the microfibrillar network. Mutation screening of 403 additional probands identified an additional missense mutation of MFAP5 (c.62G>T [p.Trp21Leu]) segregating with the disease in a second family. Functional analyses performed on both affected individual's cells and in vitro models showed that these two mutations caused pure or partial haploinsufficiency. Thus, alteration of MAGP-2, a component of microfibrils and elastic fibers, appears as an initiating mechanism of inherited TAAD.
Copyright © 2014 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Milewicz D.M., Guo D.C., Tran-Fadulu V., Lafont A.L., Papke C.L., Inamoto S., Kwartler C.S., Pannu H. Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu. Rev. Genomics Hum. Genet. 2008;9:283–302. - PubMed
-
- Jondeau G., Boileau C. Genetics of thoracic aortic aneurysms. Curr. Atheroscler. Rep. 2012;14:219–226. - PubMed
-
- Faivre L., Collod-Beroud G., Loeys B.L., Child A., Binquet C., Gautier E., Callewaert B., Arbustini E., Mayer K., Arslan-Kirchner M. Effect of mutation type and location on clinical outcome in 1,013 probands with Marfan syndrome or related phenotypes and FBN1 mutations: an international study. Am. J. Hum. Genet. 2007;81:454–466. - PMC - PubMed
-
- Loeys B.L., Schwarze U., Holm T., Callewaert B.L., Thomas G.H., Pannu H., De Backer J.F., Oswald G.L., Symoens S., Manouvrier S. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N. Engl. J. Med. 2006;355:788–798. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

