β, β-Dimethylacrylshikonin induces mitochondria-dependent apoptosis of human lung adenocarcinoma cells in vitro via p38 pathway activation
- PMID: 25434989
- PMCID: PMC4571312
- DOI: 10.1038/aps.2014.108
β, β-Dimethylacrylshikonin induces mitochondria-dependent apoptosis of human lung adenocarcinoma cells in vitro via p38 pathway activation
Abstract
Aim: β, β-Dimethylacrylshikonin (DMAS) is an anticancer compound extracted from the roots of Lithospermum erythrorhizon. In the present study, we investigated the effects of DMAS on human lung adenocarcinoma cells in vitro and explored the mechanisms of its anti-cancer action.
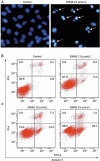
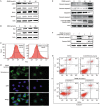
Methods: Human lung adenocarcinoma A549 cells were tested. Cell viability was assessed using an MTT assay, and cell apoptosis was evaluated with flow cytometry and DAPI staining. The expression of the related proteins was detected using Western blotting. The mitochondrial membrane potential was measured using a JC-1 kit, and subcellular distribution of cytochrome c was analyzed using immunofluorescence staining.
Results: Treatment of A549 cells with DMAS suppressed the cell viability in dose- and time-dependent manners (the IC50 value was 14.22 and 10.61 μmol/L, respectively, at 24 and 48 h). DMAS (7.5, 10, and 15 μmol/L) dose-dependently induced apoptosis, down-regulated cIAP-2 and XIAP expression, and up-regulated Bax and Bak expression in the cells. Furthermore, DMAS resulted in loss of mitochondrial membrane potential and release of cytochrome c in the cells, and activated caspase-9, caspase-8, and caspase-3, and subsequently cleaved PARP, which was abolished by pretreatment with Z-VAD-FMK, a pan-caspase inhibitor. DMAS induced sustained p38 phosphorylation in the cells, while pretreatment with SB203580, a specific p38 inhibitor, blocked DMAS-induced p38 activation and apoptosis.
Conclusion: DMAS inhibits the growth of human lung adenocarcinoma A549 cells in vitro via activation of p38 signaling pathway.
Figures



References
-
- Hanagiri T, Baba T, So T, Yasuda M, Sugaya M, Ono K, et al. Time trends of surgical outcome of patients with non-small cell lung cancer. J Thorac Oncol 2010; 5: 825–9. - PubMed
-
- Xuan Y, Hu X. Naturally-occurring shikonin analogues — a class of necroptotic inducers that circumvent cancer drug resistance. Cancer Lett 2009; 274: 233–42. - PubMed
-
- Shen CC, Syu WJ, Li SY, Lin CH, Lee GH, Sun CM. Antimicrobial activities of naphthazarins from Arnebia euchroma. J Nat Prod 2002; 65: 1857–62. - PubMed
-
- Tanaka S, Tajima M, Tsukada M, Tabata M. A comparative study on anti-inflammatory activities of the enantiomers, shikonin and alkannin. J Nat Prod 1986; 49: 466–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

