Nuclear deformability constitutes a rate-limiting step during cell migration in 3-D environments
- PMID: 25436017
- PMCID: PMC4243304
- DOI: 10.1007/s12195-014-0342-y
Nuclear deformability constitutes a rate-limiting step during cell migration in 3-D environments
Abstract
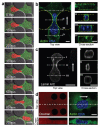
Cell motility plays a critical role in many physiological and pathological settings, ranging from wound healing to cancer metastasis. While cell migration on 2-dimensional (2-D) substrates has been studied for decades, the physical challenges cells face when moving in 3-D environments are only now emerging. In particular, the cell nucleus, which occupies a large fraction of the cell volume and is normally substantially stiffer than the surrounding cytoplasm, may impose a major obstacle when cells encounter narrow constrictions in the interstitial space, the extracellular matrix, or small capillaries. Using novel microfluidic devices that allow observation of cells moving through precisely defined geometries at high spatial and temporal resolution, we determined nuclear deformability as a critical factor in the cells' ability to pass through constrictions smaller than the size of the nucleus. Furthermore, we found that cells with reduced levels of the nuclear envelope proteins lamins A/C, which are the main determinants of nuclear stiffness, passed significantly faster through narrow constrictions during active migration and passive perfusion. Given recent reports that many human cancers have altered lamin expression, our findings suggest a novel biophysical mechanism by which changes in nuclear structure and composition may promote cancer cell invasion and metastasis.
Keywords: Lamin; cancer; invasion; mechanics; metastasis; microfluidics; microstructures; nuclear envelope; nucleus.
Figures




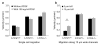

References
-
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–64. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

