Quality-of-life (QoL) as a predictive biomarker in patients with advanced pancreatic cancer (APC) receiving chemotherapy: results from a prospective multicenter phase 2 trial
- PMID: 25436122
- PMCID: PMC4226818
- DOI: 10.3978/j.issn.2078-6891.2014.070
Quality-of-life (QoL) as a predictive biomarker in patients with advanced pancreatic cancer (APC) receiving chemotherapy: results from a prospective multicenter phase 2 trial
Abstract
Purpose: Pancreatic cancer is rapidly fatal with median survival of only 6 months (mo). Quality-of-life (QoL) was analyzed prospectively in a phase 2 study of gemcitabine (G), capecitabine (C) and bevacizumab (B) in APC patients.
Methods: A total of 50 patients with APC received B 15 mg/kg, C 1,300 mg/m(2) daily for 2 weeks and G 1,000 mg/m(2) weekly 2 times; cycles were repeated every 21 days.
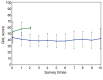
Endpoints: progression free survival (PFS), overall survival (OS) and assessment of QoL prior to each cycle using the European organization for research and treatment of cancer (EORTC) PAN-26 QoL questionnaire. An exact 95% confidence interval (CI) (Clopper-Pearson method) was used to assess rate of improved QoL (defined as >5% decrease in two consecutive scores compared with baseline).
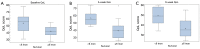
Results: Patient characteristics- Stage IIB/III/IV: 3/5/42; Sex: 28 M/22 F; Median age: 64 years. QoL in patients- improved: 56%, no improvement: 24%; unevaluable: 20%. Median PFS: 5.8 mo, OS: 9.8 mo. QoL improvement rate: 28/40=0.7 (95% CI: 0.53-0.83) in evaluable patients. Using QoL improvement rate, no significant difference was seen in patients with OS ≥6 mo compared to OS <6 mo. However QoL scores at 3 and 6 weeks from start of treatment correlated strongly with ≥6 mo survival (P value 0.0092 and 0.0081, respectively).
Conclusions: Baseline score and change in QoL scores of patients on G, C and B were not predictive of survival ≥6 mo. Post treatment scores at 3 and 6 weeks from start of therapy however, were predictive of survival ≥6 mo suggesting the potential predictive value of this tool for use in future studies.
Keywords: Quality of life (QoL); biomarkers; neoplasm, European organization for research and treatment of cancer (EORTC); outcomes; palliative care; pancreas; pancreatic cancer; supportive oncology.
Figures

References
-
- American Cancer Society. Cancer Statistics 2013. Available online: http://www.cancer.org/research/cancerfactsstatistics/
-
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. - PubMed
-
- Winter JM, Yeo CJ, Brody JR. Diagnostic, prognostic, and predictive biomarkers in pancreatic cancer. J Surg Oncol 2013;107:15-22. - PubMed
LinkOut - more resources
Full Text Sources
