The proapoptotic protein BNIP3 interacts with VDAC to induce mitochondrial release of endonuclease G
- PMID: 25436615
- PMCID: PMC4249980
- DOI: 10.1371/journal.pone.0113642
The proapoptotic protein BNIP3 interacts with VDAC to induce mitochondrial release of endonuclease G
Abstract
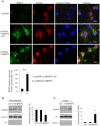
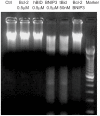
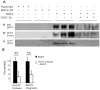
BNIP3 is a proapoptotic protein that induces cell death through a mitochondria-mediated pathway. We reported previously that mitochondrial localization of BNIP3 and translocation of EndoG from mitochondria to the nucleus are critical steps of the BNIP3 pathway. It is not clear, however, that how BNIP3 interacts with mitochondria. Here we show that expression of BNIP3 resulted in mitochondrial release and nuclear translocation of EndoG. Incubation of a recombinant GST-BNIP3 protein with freshly isolated mitochondria led to the integration of BNIP3 into mitochondria, reduction in the levels of EndoG in mitochondria and the presence of EndoG in the supernatant that was able to cleave chromatin DNA. Co-immunoprecipitation and mass spectrometry analysis reveals that BNIP3 interacted with the voltage-dependent anion channel (VDAC) to increase opening probabilities of mitochondrial permeability transition (PT) pores and induce mitochondrial release of EndoG. Blocking VDAC with a VDAC antibody largely abolished mitochondrial localization of BNIP3 and prevented EndoG release. Together, the data identify VDAC as an interacting partner of BNIP3 and support endonuclease G as a mediator of the BNIP3 pathway.
Conflict of interest statement
Figures


Similar articles
-
Mitochondrial BNIP3 upregulation precedes endonuclease G translocation in hippocampal neuronal death following oxygen-glucose deprivation.BMC Neurosci. 2009 Sep 8;10:113. doi: 10.1186/1471-2202-10-113. BMC Neurosci. 2009. PMID: 19737385 Free PMC article.
-
BNIP3 upregulation and EndoG translocation in delayed neuronal death in stroke and in hypoxia.Stroke. 2007 May;38(5):1606-13. doi: 10.1161/STROKEAHA.106.475129. Epub 2007 Mar 22. Stroke. 2007. PMID: 17379825
-
EndoG links Bnip3-induced mitochondrial damage and caspase-independent DNA fragmentation in ischemic cardiomyocytes.PLoS One. 2011 Mar 17;6(3):e17998. doi: 10.1371/journal.pone.0017998. PLoS One. 2011. PMID: 21437288 Free PMC article.
-
Communication between mitochondria and nucleus: putative role for VDAC in reduction/oxidation mechanism.Biochim Biophys Acta. 2010 Jun-Jul;1797(6-7):1276-80. doi: 10.1016/j.bbabio.2010.02.004. Epub 2010 Feb 6. Biochim Biophys Acta. 2010. PMID: 20144586 Review.
-
New findings concerning vertebrate porin II--on the relevance of glycine motifs of type-1 VDAC.Mol Genet Metab. 2013 Apr;108(4):212-24. doi: 10.1016/j.ymgme.2013.01.008. Epub 2013 Jan 26. Mol Genet Metab. 2013. PMID: 23419876 Review.
Cited by
-
VDAC1 at the crossroads of cell metabolism, apoptosis and cell stress.Cell Stress. 2017 Oct;1(1):11-36. doi: 10.15698/cst2017.10.104. Epub 2017 Oct 1. Cell Stress. 2017. PMID: 30542671 Free PMC article.
-
Meiotic viral attenuation through an ancestral apoptotic pathway.Proc Natl Acad Sci U S A. 2019 Aug 13;116(33):16454-16462. doi: 10.1073/pnas.1900751116. Epub 2019 Jul 2. Proc Natl Acad Sci U S A. 2019. PMID: 31266891 Free PMC article.
-
Modes of Cell Death Induced by Photodynamic Therapy Using Zinc Phthalocyanine in Lung Cancer Cells Grown as a Monolayer and Three-Dimensional Multicellular Spheroids.Molecules. 2017 May 16;22(5):791. doi: 10.3390/molecules22050791. Molecules. 2017. PMID: 28509858 Free PMC article.
-
VDAC1 at the Intersection of Cell Metabolism, Apoptosis, and Diseases.Biomolecules. 2020 Oct 26;10(11):1485. doi: 10.3390/biom10111485. Biomolecules. 2020. PMID: 33114780 Free PMC article. Review.
-
ATP functions as a primary alarmin in allergen-induced type 2 immunity.Am J Physiol Cell Physiol. 2023 Nov 1;325(5):C1369-C1386. doi: 10.1152/ajpcell.00370.2023. Epub 2023 Oct 16. Am J Physiol Cell Physiol. 2023. PMID: 37842751 Free PMC article. Review.
References
-
- Boyd JM, Malstrom S, Subramanian T, Venkatesh LK, Schaeper U, et al. (1994) Adenovirus E1B 19 kDa and Bcl-2 proteins interact with a common set of cellular proteins. . Cell 79:341–351. - PubMed
-
- Erkan M, Kleeff J, Esposito I, Giese T, Ketterer K, et al. (2005) Loss of BNIP3 expression is a late event in pancreatic cancer contributing to chemoresistance and worsened prognosis. . Oncogene 24:4421–4432. - PubMed
-
- Li C, Guan T, Chen X, Li W, Cai Q, et al. (2013) BNIP3 mediates pre-myelinating oligodendrocyte cell death in hypoxia and ischemia. . J Neurochem 127:426–433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

