Poly I:C adjuvanted inactivated swine influenza vaccine induces heterologous protective immunity in pigs
- PMID: 25437101
- PMCID: PMC7115561
- DOI: 10.1016/j.vaccine.2014.11.034
Poly I:C adjuvanted inactivated swine influenza vaccine induces heterologous protective immunity in pigs
Abstract
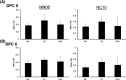
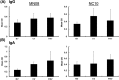
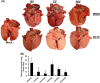
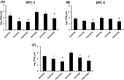
Swine influenza is widely prevalent in swine herds in North America and Europe causing enormous economic losses and a public health threat. Pigs can be infected by both avian and mammalian influenza viruses and are sources of generation of reassortant influenza viruses capable of causing pandemics in humans. Current commercial vaccines provide satisfactory immunity against homologous viruses; however, protection against heterologous viruses is not adequate. In this study, we evaluated the protective efficacy of an intranasal Poly I:C adjuvanted UV inactivated bivalent swine influenza vaccine consisting of Swine/OH/24366/07 H1N1 and Swine/CO/99 H3N2, referred as PAV, in maternal antibody positive pigs against an antigenic variant and a heterologous swine influenza virus challenge. Groups of three-week-old commercial-grade pigs were immunized intranasally with PAV or a commercial vaccine (CV) twice at 2 weeks intervals. Three weeks after the second immunization, pigs were challenged with the antigenic variant Swine/MN/08 H1N1 (MN08) and the heterologous Swine/NC/10 H1N2 (NC10) influenza virus. Antibodies in serum and respiratory tract, lung lesions, virus shedding in nasal secretions and virus load in lungs were assessed. Intranasal administration of PAV induced challenge viruses specific-hemagglutination inhibition- and IgG antibodies in the serum and IgA and IgG antibodies in the respiratory tract. Importantly, intranasal administration of PAV provided protection against the antigenic variant MN08 and the heterologous NC10 swine influenza viruses as evidenced by significant reductions in lung virus load, gross lung lesions and significantly reduced shedding of challenge viruses in nasal secretions. These results indicate that Poly I:C or its homologues may be effective as vaccine adjuvants capable of generating cross-protective immunity against antigenic variants/heterologous swine influenza viruses in pigs.
Keywords: Inactivated swine influenza vaccines; Poly I:C; Swine influenza virus; Vaccine adjuvants.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
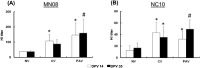
References
-
- Vincent A., Awada L., Brown I., Chen H., Claes F., Dauphin G. Review of influenza A virus in swine worldwide: a call for increased surveillance and research. Zoonoses Public Health. 2014;61(1 (Feb)):4–17. - PubMed
-
- Hause B.M., Oleson T.A., Bey R.F., Stine D.L., Simonson R.R. Antigenic categorization of contemporary H3N2 Swine influenza virus isolates using a high-throughput serum neutralization assay. J Vet Diagn Investig. 2010;22(3 (May)):352–359. - PubMed
-
- Kitikoon P., Gauger P.C., Anderson T.K., Culhane M.R., Swenson S., Loving C.L. Swine influenza virus vaccine serologic cross-reactivity to contemporary US swine H3N2 and efficacy in pigs infected with an H3N2 similar to 2011–2012 H3N2v. Influenza Other Respir Viruses. 2013;7(4 (Dec)):32–41. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

