Alzheimer Disease Biomarkers as Outcome Measures for Clinical Trials in MCI
- PMID: 25437302
- PMCID: PMC4437812
- DOI: 10.1097/WAD.0000000000000071
Alzheimer Disease Biomarkers as Outcome Measures for Clinical Trials in MCI
Abstract
Background: The aim of this study was to compare the performance and power of the best-established diagnostic biological markers as outcome measures for clinical trials in patients with mild cognitive impairment (MCI).
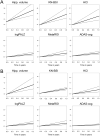
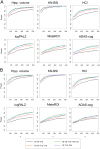
Methods: Magnetic resonance imaging, F-18 fluorodeoxyglucose positron emission tomography markers, and Alzheimer's Disease Assessment Scale-cognitive subscale were compared in terms of effect size and statistical power over different follow-up periods in 2 MCI groups, selected from Alzheimer's Disease Neuroimaging Initiative data set based on cerebrospinal fluid (abnormal cerebrospinal fluid Aβ1-42 concentration-ABETA+) or magnetic resonance imaging evidence of Alzheimer disease (positivity to hippocampal atrophy-HIPPO+). Biomarkers progression was modeled through mixed effect models. Scaled slope was chosen as measure of effect size. Biomarkers power was estimated using simulation algorithms.
Results: Seventy-four ABETA+ and 51 HIPPO+ MCI patients were included in the study. Imaging biomarkers of neurodegeneration, especially MR measurements, showed highest performance. For all biomarkers and both MCI groups, power increased with increasing follow-up time, irrespective of biomarker assessment frequency.
Conclusion: These findings provide information about biomarker enrichment and outcome measurements that could be employed to reduce MCI patient samples and treatment duration in future clinical trials.
Conflict of interest statement
Figures
References
-
- Selkoe DJ. Resolving controversies on the path to Alzheimer's therapeutics. Nat Med. 2011;17:1060–1065. - PubMed
-
- Selkoe DJ. Preventing Alzheimer's disease. Science. 2012;337:1488–1492. - PubMed
-
- Lorenzi M, Donohue M, Paternicò D, et al. Alzheimer's Disease Neuroimaging Initiative. Enrichment through biomarkers in clinical trials of Alzheimer's drugs in patients with mild cognitive impairment. Neurobiol Aging. 2010;31:1443–1451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

