Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis
- PMID: 25437556
- PMCID: PMC4250841
- DOI: 10.1016/j.celrep.2014.09.049
Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis
Abstract
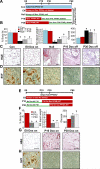
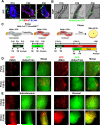
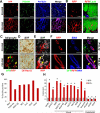
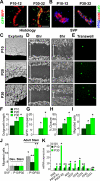
Adipose tissues have striking plasticity, highlighted by childhood and adult obesity. Using adipose lineage analyses, smooth muscle actin (SMA)-mural cell-fate mapping, and conditional PPARγ deletion to block adipocyte differentiation, we find two phases of adipocyte generation that emanate from two independent adipose progenitor compartments: developmental and adult. These two compartments are sequentially required for organ formation and maintenance. Although both developmental and adult progenitors are specified during the developmental period and express PPARγ, they have distinct microanatomical, functional, morphogenetic, and molecular profiles. Furthermore, the two compartments derive from different lineages; whereas adult adipose progenitors fate-map from an SMA+ mural lineage, developmental progenitors do not. Remarkably, the adult progenitor compartment appears to be specified earlier than the developmental cells and then enters the already developmentally formed adipose depots. Thus, two distinct cell compartments control adipose organ development and organ homeostasis, which may provide a discrete therapeutic target for childhood and adult obesity.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Ailhaud G, Grimaldi P, Negrel R. Cellular and molecular aspects of adipose tissue development. Annu Rev Nutr. 1992;12:207–233. - PubMed
-
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

