Glycomic analysis of membrane glycoproteins with bisecting glycosylation from ovarian cancer tissues reveals novel structures and functions
- PMID: 25437919
- PMCID: PMC4286206
- DOI: 10.1021/pr501174p
Glycomic analysis of membrane glycoproteins with bisecting glycosylation from ovarian cancer tissues reveals novel structures and functions
Abstract
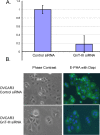
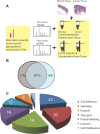
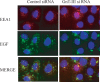
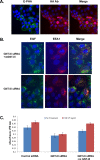
Biomarkers capable of detecting and targeting epithelial ovarian cancer cells for diagnostics and therapeutics would be extremely valuable. Ovarian cancer is the deadliest reproductive malignancy among women in the U.S., killing over 14 000 women each year. Both the lack of presenting symptoms and high mortality rates illustrate the need for earlier diagnosis and improved treatment of this disease. The glycosyltransferase enzyme GnT-III encoded by the Mgat3 gene is responsible for the addition of GlcNAc (N-acetylglucosamine) to form bisecting N-linked glycan structures. GnT-III mRNA expression is amplified in ovarian cancer tissues compared with normal ovarian tissue. We use a lectin capture strategy coupled to nano-ESI-RPLC-MS/MS to isolate and identify the membrane glycoproteins and unique glycan structures associated with GnT-III amplification in human ovarian cancer tissues. Our data illustrate that the majority of membrane glycoproteins with bisecting glycosylation are common to both serous and endometrioid histological subtypes of ovarian cancer, and several have been reported to participate in signaling pathways such as Notch, Wnt, and TGFβ.
Keywords: biomarkers; bisecting N-linked glycans; glycoproteins; human tissue; mass spectrometry; ovarian cancer.
Figures


References
-
- Bhaumik M.; Seldin M. F.; Stanley P. Cloning and chromosomal mapping of the mouse Mgat3 gene encoding N-acetylglucosaminyltransferase III. Gene 1995, 1642295–300. - PubMed
-
- Koles K.; et al. Identification of N-glycosylated proteins from the central nervous system of Drosophila melanogaster. Glycobiology 2007, 17121388–1403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

