A bicyclic peptide scaffold promotes phosphotyrosine mimicry and cellular uptake
- PMID: 25438762
- PMCID: PMC4254597
- DOI: 10.1016/j.bmc.2014.09.050
A bicyclic peptide scaffold promotes phosphotyrosine mimicry and cellular uptake
Abstract
While peptides are promising as probes and therapeutics, targeting intracellular proteins will require greater understanding of highly structured, cell-internalized scaffolds. We recently reported BC1, an 11-residue bicyclic peptide that inhibits the Src homology 2 (SH2) domain of growth factor receptor-bound protein 2 (Grb2). In this work, we describe the unique structural and cell uptake properties of BC1 and similar cyclic and bicyclic scaffolds. These constrained scaffolds are taken up by mammalian cells despite their net neutral or negative charges, while unconstrained analogs are not. The mechanism of uptake is shown to be energy-dependent and endocytic, but distinct from that of Tat. The solution structure of BC1 was investigated by NMR and MD simulations, which revealed discrete water-binding sites on BC1 that reduce exposure of backbone amides to bulk water. This represents an original and potentially general strategy for promoting cell uptake.
Keywords: Bicyclic peptide; Cell uptake; Cyclic peptide; Molecular dynamics; NMR structure; Phosphotyrosine.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

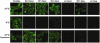
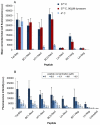
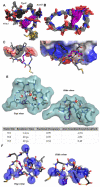
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

