3-iodothyroacetic acid, a metabolite of thyroid hormone, induces itch and reduces threshold to noxious and to painful heat stimuli in mice
- PMID: 25439265
- PMCID: PMC4376462
- DOI: 10.1111/bph.13032
3-iodothyroacetic acid, a metabolite of thyroid hormone, induces itch and reduces threshold to noxious and to painful heat stimuli in mice
Abstract
Background and purpose: Itch is associated with increased sensitization to nociceptive stimuli. We investigated whether 3-iodothyroacetic acid (TA1), by releasing histamine, induces itch and increases sensitization to noxious and painful heat stimuli.
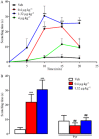
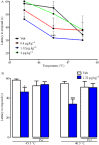
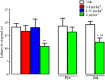
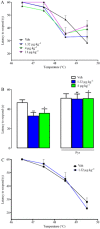
Experimental approach: Itch was evaluated after s.c. administration of TA1 (0.4, 1.32 and 4 μg·kg(-1) ). Mice threshold to noxious (NHT) and to painful heat stimuli were evaluated by the increasing-temperature hot plate (from 45.5 to 49.5°C) or by the hot plate (51.5°C) test, respectively, 15 min after i.p. injection of TA1 (0.4, 1.32 and 4 μg·kg(-1) ). Itch, NHT and pain threshold evaluation were repeated in mice pretreated with pyrilamine. Itch and NHT were also measured in HDC(+/+) and HDC(-/-) following injection of saline or TA1 (1.32, 4 and 11 μg·kg(-1) ; s.c. and i.p.). pERK1/2 levels were determined by Western blot in dorsal root ganglia (DRG) isolated from CD1 mice 15 min after they received (i.p.): saline, saline and noxious heat stimulus (46.5°C), TA1 (0.1, 0.4, 1.32, 4 μg·kg(-1) ) or TA1 1.32 μg·kg(-1) and noxious heat stimulus.
Key results: TA1 0.4 and 1.32 μg·kg(-1) induced itch and reduced NHT; pyrilamine pretreatment prevented both of these effects. TA1 4 μg·kg(-1) (i.p.) reduced pain threshold without inducing itch or modifying NHT. In HDC(-/-) mice, TA1 failed to induce itch and to reduce NHT. In DRG, pERK1/2 levels were significantly increased by noxious heat stimuli and by TA1 0.1, 0.4 and 1.32 μg·kg(-1) ; i.p.
Conclusions and implications: Increased TA1 levels induce itch and an enhanced sensitivity to noxious heat stimuli suggesting that TA1 might represent a potential cause of itch in thyroid diseases.
© 2014 The British Pharmacological Society.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

