Sigma receptor 1 activation attenuates release of inflammatory cytokines MIP1γ, MIP2, MIP3α, and IL12 (p40/p70) by retinal Müller glial cells
- PMID: 25439327
- PMCID: PMC4451448
- DOI: 10.1111/jnc.13002
Sigma receptor 1 activation attenuates release of inflammatory cytokines MIP1γ, MIP2, MIP3α, and IL12 (p40/p70) by retinal Müller glial cells
Abstract
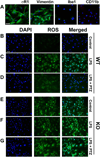
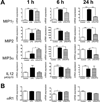
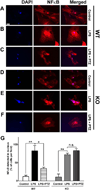
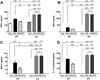
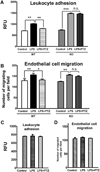
The high-affinity sigma receptor 1 (σR1) ligand (+)-pentazocine ((+)-PTZ) affords profound retinal neuroprotection in vitro and in vivo by a yet-unknown mechanism. A common feature of retinal disease is Müller cell reactive gliosis, which includes cytokine release. Here, we investigated whether lipopolysaccharide (LPS) stimulates cytokine release by primary mouse Müller cells and whether (+)-PTZ alters release. Using a highly sensitive inflammatory antibody array we observed significant release of macrophage inflammatory proteins (MIP1γ, MIP2, MIP3α) and interleukin-12 (IL12 (p40/p70)) in LPS-treated cells compared to controls, and a significant decrease in secretion upon (+)-PTZ treatment. Müller cells from σR1 knockout mice demonstrated increased MIP1γ, MIP2, MIP3α and IL12 (p40/p70) secretion when exposed to LPS compared to LPS-stimulated WT cells. We investigated whether cytokine secretion was accompanied by cytosolic-to-nuclear NFκB translocation and whether endothelial cell adhesion/migration was altered by released cytokines. Cells exposed to LPS demonstrated increased NFκB nuclear location, which was reduced significantly in (+)-PTZ-treated cells. Media conditioned by LPS-stimulated-Müller cells induced leukocyte-endothelial cell adhesion and endothelial cell migration, which was attenuated by (+)-PTZ treatment. The findings suggest that release of certain inflammatory cytokines by Müller cells can be attenuated by σR1 ligands providing insights into the retinal neuroprotective role of this receptor.
Keywords: (+)-pentazocine; cytokine array; mouse; radial glial cell; retinal degeneration; retinal disease.
© 2014 International Society for Neurochemistry.
Figures

References
-
- Armstrong DA, Major JA, Chudyk A, Hamilton TA. Neutrophil chemoattractant genes KC and MIP-2 are expressed in different cell populations at sites of surgical injury. J Leukoc Biol. 2004;75:641–648. - PubMed
-
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. - PubMed
-
- Biedermann T, Kneilling M, Mailhammer R, Maier K, Sander CA, Kollias G, Kunkel SL, Hültner L, Röcken M. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J Exp Med. 2000;192:1441–1452. - PMC - PubMed
-
- Behensky AA, Yasny IE, Shuster AM, Seredenin SB, Petrov AV, Cuevas J. Stimulation of sigma receptors with afobazole blocks activation of microglia and reduces toxicity caused by amyloid-β25–35. J Pharmacol Exp Ther. 2013;347:458–467. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

