Intrafollicular cortisol levels inversely correlate with cumulus cell lipid content as a possible energy source during oocyte meiotic resumption in women undergoing ovarian stimulation for in vitro fertilization
- PMID: 25439840
- PMCID: PMC4380141
- DOI: 10.1016/j.fertnstert.2014.09.034
Intrafollicular cortisol levels inversely correlate with cumulus cell lipid content as a possible energy source during oocyte meiotic resumption in women undergoing ovarian stimulation for in vitro fertilization
Abstract
Objective: To determine whether follicular fluid (FF) cortisol levels affect cumulus cell (CC) lipid content during oocyte meiotic resumption, and whether CCs express genes for glucocorticoid action.
Design: Prospective cohort study.
Setting: Academic medical center.
Patient(s): Thirty-seven nonobese women underwent ovarian stimulation for in vitro fertilization (IVF).
Intervention(s): At oocyte retrieval, FF was aspirated from the first follicle (>16 mm in size) of each ovary and pooled CCs were collected.
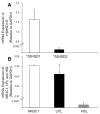
Main outcome measure(s): Follicular fluid cortisol and cortisone analysis was performed with the use of liquid chromatography-tandem mass spectrometry. CCs were stained with lipid fluorescent dye Bodipy FL C16 to determine lipid content with the use of confocal microscopy. Quantitative real-time polymerase chain reaction was used to detect CC gene expression of 11β-hydroxysteroid dehydrogenase (11β-HSD) types 1 and 2, glucocorticoid receptor (NR3C1), lipoprotein lipase (LPL), and hormone-sensitive lipase (HSL).
Result(s): Adjusting for maternal age, FF cortisol levels negatively correlated with CC lipid content and positively correlated with numbers of total and mature oocytes. CCs expressed genes for 11β-HSD type 1 as the predominant 11β-HSD isoform, NR3C1, LPL, and HSL.
Conclusion(s): FF cortisol levels may regulate CC lipolysis during oocyte meiotic resumption and affect oocyte quality during IVF.
Keywords: Cortisol; cumulus cell lipid; in vitro fertilization; meiosis; oocyte developmental competence.
Copyright © 2015 American Society for Reproductive Medicine. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Dunning KR, Cashman K, Russell DL, Thompson JG, Norman RJ, Robker RL. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biol Reprod. 2010;83(6):909–18. - PubMed
-
- Dunning KR, Robker RL. Promoting lipid utilization with l-carnitine to improve oocyte quality. Anim Reprod Sci. 2012;134(1–2):69–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

