The LXR-Idol axis differentially regulates plasma LDL levels in primates and mice
- PMID: 25440061
- PMCID: PMC4261644
- DOI: 10.1016/j.cmet.2014.10.001
The LXR-Idol axis differentially regulates plasma LDL levels in primates and mice
Abstract
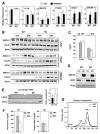
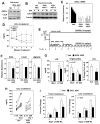
The LXR-regulated E3 ubiquitin ligase IDOL controls LDLR receptor stability independent of SREBP and PCSK9, but its relevance to plasma lipid levels is unknown. Here we demonstrate that the effects of the LXR-IDOL axis are both tissue and species specific. In mice, LXR agonist induces Idol transcript levels in peripheral tissues but not in liver, and does not change plasma LDL levels. Accordingly, Idol-deficient mice exhibit elevated LDLR protein levels in peripheral tissues, but not in the liver. By contrast, LXR activation in cynomolgus monkeys induces hepatic IDOL expression, reduces LDLR protein levels, and raises plasma LDL levels. Knockdown of IDOL in monkeys with an antisense oligonucleotide blunts the effect of LXR agonist on LDL levels. These results implicate IDOL as a modulator of plasma lipid levels in primates and support further investigation into IDOL inhibition as a potential strategy for LDL lowering in humans.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


References
-
- Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. - PubMed
-
- Agellon LB, Walsh A, Hayek T, Moulin P, Jiang XC, Shelanski SA, Breslow JL, Tall AR. Reduced high density lipoprotein cholesterol in human cholesteryl ester transfer protein transgenic mice. J Biol Chem. 1991;266:10796–10801. - PubMed
-
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. - PubMed
-
- Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL090553/HL/NHLBI NIH HHS/United States
- HL088528/HL/NHLBI NIH HHS/United States
- HL066088/HL/NHLBI NIH HHS/United States
- P01 HL090553/HL/NHLBI NIH HHS/United States
- R01 HL111932/HL/NHLBI NIH HHS/United States
- HL111932/HL/NHLBI NIH HHS/United States
- T32 HL091797/HL/NHLBI NIH HHS/United States
- P30 DK041301/DK/NIDDK NIH HHS/United States
- R01 HL066088/HL/NHLBI NIH HHS/United States
- Howard Hughes Medical Institute/United States
- R00 HL088528/HL/NHLBI NIH HHS/United States
- K99 HL088528/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

