Antiplasmodial activity of chloroquine analogs against chloroquine-resistant parasites, docking studies and mechanisms of drug action
- PMID: 25440372
- PMCID: PMC4265395
- DOI: 10.1186/1475-2875-13-469
Antiplasmodial activity of chloroquine analogs against chloroquine-resistant parasites, docking studies and mechanisms of drug action
Abstract
Background: Given the threat of resistance of human malaria parasites, including to artemisinin derivatives, new agents are needed. Chloroquine (CQ) has been the most widely used anti-malarial, and new analogs (CQAns) presenting alkynes and side chain variations with high antiplasmodial activity were evaluated.
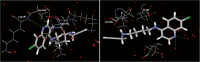
Methods: Six diaminealkyne and diaminedialkyne CQAns were evaluated against CQ-resistant (CQ-R) (W2) and CQ-sensitive (CQ-S) (3D7) Plasmodium falciparum parasites in culture. Drug cytotoxicity to a human hepatoma cell line (HepG2) evaluated, allowed to calculate the drug selectivity index (SI), a ratio of drug toxicity to activity in vitro. The CQAns were re-evaluated against CQ-resistant and -sensitive P. berghei parasites in mice using the suppressive test. Docking studies with the CQAns and the human (HssLDH) or plasmodial lactate dehydrogenase (PfLDH) enzymes, and, a β-haematin formation assay were performed using a lipid as a catalyst to promote crystallization in vitro.
Results: All tested CQAns were highly active against CQ-R P. falciparum parasites, exhibiting half-maximal inhibitory concentration (IC(50)) values below 1 μΜ. CQAn33 and CQAn37 had the highest SIs. Docking studies revealed the best conformation of CQAn33 inside the binding pocket of PfLDH; specificity between the residues involved in H-bonds of the PfLDH with CQAn37. CQAn33 and CQAn37 were also shown to be weak inhibitors of PfLDH. CQAn33 and CQAn37 inhibited β-haematin formation with either a similar or a 2-fold higher IC(50) value, respectively, compared with CQ. CQAn37 was active in mice with P. berghei, reducing parasitaemia by 100%. CQAn33, -39 and -45 also inhibited CQ-resistant P. berghei parasites in mice, whereas high doses of CQ were inactive.
Conclusions: The presence of an alkyne group and the size of the side chain affected anti-P. falciparum activity in vitro. Docking studies suggested a mechanism of action other than PfLDH inhibition. The β-haematin assay suggested the presence of an additional mechanism of action of CQAn33 and CQAn37. Tests with CQAn34, CQAn37, CQAn39 and CQAn45 confirmed previous results against P. berghei malaria in mice, and CQAn33, 39 and 45 were active against CQ-resistant parasites, but CQAn28 and CQAn34 were not. The result likely reflects structure-activity relationships related to the resistant phenotype.
Figures


References
-
- WHO . World Malaria Report 2013. Geneva: World Health Organization; 2014.
-
- WHO . Guidelines for Treatment of Human Malaria. 2. Geneva: World Health Organization; 2010.
-
- WHO . Global Report on Antimalarial Drug Efficacy and Drug Resistance 2000–2010. Geneva: World Health Organization; 2010.
-
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–457. doi: 10.1056/NEJMoa0808859. - DOI - PMC - PubMed
-
- Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, ler Moo C, Al-Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NP, White NJ, Anderson TJ, Nosten F. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379:1960–1966. doi: 10.1016/S0140-6736(12)60484-X. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

