From monoclonal antibodies to chimeric antigen receptors for the treatment of human malignancies
- PMID: 25440610
- PMCID: PMC4254449
- DOI: 10.1053/j.seminoncol.2014.08.005
From monoclonal antibodies to chimeric antigen receptors for the treatment of human malignancies
Abstract
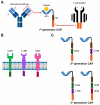
Monoclonal antibodies (mAbs) and their directly derived cell-based application known as chimeric antigen receptors (CARs) ensue from the need to develop novel therapeutic strategies that retain high anti-tumor activity, but carry reduced toxicity compared to conventional chemo- and radiotherapies. In this concise review article, we will summarize the application of antibodies designed to target antigens expressed by tumor cells, and the transition from these antibodies to the generation of CARs.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
The pharmacology of second-generation chimeric antigen receptors.Nat Rev Drug Discov. 2015 Jul;14(7):499-509. doi: 10.1038/nrd4597. Nat Rev Drug Discov. 2015. PMID: 26129802 Free PMC article. Review.
-
Advanced generation anti-prostate specific membrane antigen designer T cells for prostate cancer immunotherapy.Prostate. 2014 Feb;74(3):286-96. doi: 10.1002/pros.22749. Prostate. 2014. PMID: 24174378
-
Improving the efficacy and safety of engineered T cell therapy for cancer.Cancer Lett. 2013 Jan 28;328(2):191-7. doi: 10.1016/j.canlet.2012.09.015. Epub 2012 Sep 27. Cancer Lett. 2013. PMID: 23022475 Review.
-
Cancer gene therapy with T cell receptors and chimeric antigen receptors.Curr Opin Pharmacol. 2015 Oct;24:113-8. doi: 10.1016/j.coph.2015.08.006. Epub 2015 Sep 4. Curr Opin Pharmacol. 2015. PMID: 26342910 Review.
-
Using monoclonal antibodies to stimulate antitumor cellular immunity.Expert Rev Vaccines. 2011 Jul;10(7):1093-106. doi: 10.1586/erv.11.33. Expert Rev Vaccines. 2011. PMID: 21806402 Review.
Cited by
-
Manipulating the Metabolism to Improve the Efficacy of CAR T-Cell Immunotherapy.Cells. 2020 Dec 24;10(1):14. doi: 10.3390/cells10010014. Cells. 2020. PMID: 33374128 Free PMC article. Review.
-
Chimeric Antigen Receptor T Cell with an Inducible Caspase-9 Suicide Gene Eradicates Uveal Melanoma Liver Metastases via B7-H3 Targeting.Clin Cancer Res. 2024 Aug 1;30(15):3243-3258. doi: 10.1158/1078-0432.CCR-24-0071. Clin Cancer Res. 2024. PMID: 38767611 Free PMC article.
-
Engineered natural killer cells impede the immunometabolic CD73-adenosine axis in solid tumors.Elife. 2022 Jul 11;11:e73699. doi: 10.7554/eLife.73699. Elife. 2022. PMID: 35815945 Free PMC article.
-
The antigen-binding moiety in the driver's seat of CARs.Med Res Rev. 2022 Jan;42(1):306-342. doi: 10.1002/med.21818. Epub 2021 May 24. Med Res Rev. 2022. PMID: 34028069 Free PMC article. Review.
-
Rapid Fire: Infectious Disease Emergencies in Patients with Cancer.Emerg Med Clin North Am. 2018 Aug;36(3):493-516. doi: 10.1016/j.emc.2018.04.001. Epub 2018 Jun 11. Emerg Med Clin North Am. 2018. PMID: 30037437 Free PMC article. Review.
References
-
- Nadler LM, Stashenko P, Hardy R, Kaplan WD, Button LN, Kufe DW, Antman KH, Schlossman SF. Serotherapy of a patient with a monoclonal antibody directed against a human lymphoma-associated antigen. Cancer Res. 1980;40:3147–3154. - PubMed
-
- Cosimi AB, Colvin RB, Burton RC, Rubin RH, Goldstein G, Kung PC, Hansen WP, Delmonico FL, Russell PS. Use of monoclonal antibodies to T-cell subsets for immunologic monitoring and treatment in recipients of renal allografts. N. Engl. J. Med. 1981;305:308–314. - PubMed
-
- Oldham RK, Dillman RO. Monoclonal antibodies in cancer therapy: 25 years of progress. J. Clin. Oncol. 2008;26:1774–1777. - PubMed
-
- Maloney DG. Follicular NHL: from antibodies and vaccines to graft-versus-lymphoma effects. Hematology. Am. Soc. Hematol. Educ. Program. 2007:226–232. - PubMed
-
- Reichert JM, Valge-Archer VE. Development trends for monoclonal antibody cancer therapeutics. Nat. Rev. Drug Discov. 2007;6:349–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

