Prostaglandin D2 and leukotriene E4 synergize to stimulate diverse TH2 functions and TH2 cell/neutrophil crosstalk
- PMID: 25441644
- PMCID: PMC4418751
- DOI: 10.1016/j.jaci.2014.09.006
Prostaglandin D2 and leukotriene E4 synergize to stimulate diverse TH2 functions and TH2 cell/neutrophil crosstalk
Abstract
Background: Prostaglandin D2 (PGD2) and cysteinyl leukotrienes (cysLTs) are lipid mediators derived from mast cells, which activate TH2 cells. The combination of PGD2 and cysLTs (notably cysteinyl leukotriene E4 [LTE4]) enhances TH2 cytokine production. However, the synergistic interaction of cysLTs with PGD2 in promoting TH2 cell activation is still poorly understood. The receptors for these mediators are drug targets in the treatment of allergic diseases, and hence understanding their interaction is likely to have clinical implications.
Objective: We aimed to comprehensively define the roles of PGD2, LTE4, and their combination in activating human TH2 cells and how such activation might allow the TH2 cells to engage downstream effectors, such as neutrophils, which contribute to the pathology of allergic responses.
Methods: The effects of PGD2, LTE4, and their combination on human TH2 cell gene expression were defined by using a microarray, and changes in specific inflammatory pathways were confirmed by means of PCR array, quantitative RT-PCR, ELISA, Luminex, flow cytometry, and functional assays, including analysis of downstream neutrophil activation. Blockade of PGD2 and LTE4 was tested by using TM30089, an antagonist of chemoattractant receptor-homologous molecule expressed on TH2 cells, and montelukast, an antagonist of cysteinyl leukotriene receptor 1.
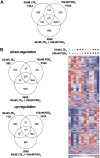
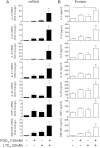
Results: PGD2 and LTE4 altered the transcription of a wide range of genes and induced diverse functional responses in TH2 cells, including cell adhesion, migration, and survival and cytokine production. The combination of these lipids synergistically or additively enhanced TH2 responses and, strikingly, induced marked production of diverse nonclassical TH2 inflammatory mediators, including IL-22, IL-8, and GM-CSF, at concentrations sufficient to affect neutrophil activation.
Conclusions: PGD2 and LTE4 activate TH2 cells through different pathways but act synergistically to promote multiple downstream effector functions, including neutrophil migration and survival. Combined inhibition of both PGD2 and LTE4 pathways might provide an effective therapeutic strategy for allergic responses, particularly those involving interaction between TH2 cells and neutrophils, such as in patients with severe asthma.
Keywords: Prostaglandin D(2); T(H)2 cells; chemoattractant receptor-homologous molecule expressed on T(H)2 cells; leukotriene E(4); neutrophils.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures










Similar articles
-
Cysteinyl leukotriene E4 activates human group 2 innate lymphoid cells and enhances the effect of prostaglandin D2 and epithelial cytokines.J Allergy Clin Immunol. 2017 Oct;140(4):1090-1100.e11. doi: 10.1016/j.jaci.2016.12.958. Epub 2017 Jan 20. J Allergy Clin Immunol. 2017. PMID: 28115217 Free PMC article.
-
Evidence for the induction of Th2 inflammation by group 2 innate lymphoid cells in response to prostaglandin D2 and cysteinyl leukotrienes in allergic rhinitis.Allergy. 2019 Dec;74(12):2417-2426. doi: 10.1111/all.13974. Epub 2019 Aug 12. Allergy. 2019. PMID: 31267527
-
Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells.J Immunol. 2005 Nov 15;175(10):6531-6. doi: 10.4049/jimmunol.175.10.6531. J Immunol. 2005. PMID: 16272307
-
Involvement and Possible Role of Eosinophils in Asthma Exacerbation.Front Immunol. 2018 Sep 28;9:2220. doi: 10.3389/fimmu.2018.02220. eCollection 2018. Front Immunol. 2018. PMID: 30323811 Free PMC article. Review.
-
CRTH2 and D-type prostanoid receptor antagonists as novel therapeutic agents for inflammatory diseases.Pharmacology. 2010;85(6):372-82. doi: 10.1159/000313836. Epub 2010 Jun 16. Pharmacology. 2010. PMID: 20559016 Review.
Cited by
-
Prostaglandins and Their Receptors in Eosinophil Function and As Therapeutic Targets.Front Med (Lausanne). 2017 Jul 19;4:104. doi: 10.3389/fmed.2017.00104. eCollection 2017. Front Med (Lausanne). 2017. PMID: 28770200 Free PMC article. Review.
-
Synergistic activation of pro-inflammatory type-2 CD8+ T lymphocytes by lipid mediators in severe eosinophilic asthma.Mucosal Immunol. 2018 Sep;11(5):1408-1419. doi: 10.1038/s41385-018-0049-9. Epub 2018 Jun 15. Mucosal Immunol. 2018. PMID: 29907870 Free PMC article.
-
Enhancer of zeste homolog 2-catalysed H3K27 trimethylation plays a key role in acute-on-chronic liver failure via TNF-mediated pathway.Cell Death Dis. 2018 May 22;9(6):590. doi: 10.1038/s41419-018-0670-2. Cell Death Dis. 2018. PMID: 29789597 Free PMC article.
-
Novel data analysis method for multicolour flow cytometry links variability of multiple markers on single cells to a clinical phenotype.Sci Rep. 2017 Jul 14;7(1):5471. doi: 10.1038/s41598-017-05714-1. Sci Rep. 2017. PMID: 28710472 Free PMC article.
-
Diminished Diversities and Clonally Expanded Sequences of T-Cell Receptors in Patients with Chronic Spontaneous Urticaria.Immunotargets Ther. 2024 Dec 5;13:661-671. doi: 10.2147/ITT.S481361. eCollection 2024. Immunotargets Ther. 2024. PMID: 39659518 Free PMC article.
References
-
- Schleimer R.P., Fox C.C., Naclerio R.M., Plaut M., Creticos P.S., Togias A.G. Role of human basophils and mast cells in the pathogenesis of allergic diseases. J Allergy Clin Immunol. 1985;76:369–374. - PubMed
-
- Philipps G.D., Holgate S.T. Interaction of inhaled LTC4 with histamine and PGD2 on airway caliber in asthma. J Appl Physiol. 1989;66:304–312. - PubMed
-
- Fujitani Y., Kanaoka Y., Aritake K., Uodome N., Okazaki-Hatake K., Urade Y. Pronounced eosinophilic lung inflammation and Th2 cytokine release in human lipocalin-type prostaglandin D synthase transgenic mice. J Immunol. 2000;168:443–449. - PubMed
-
- Pettipher R., Hansel T.T., Armer R. Antagonism of the prostaglandin D2 receptors DP1 and CRTH2 as an approach to treat allergic diseases. Nat Rev Drug Discov. 2007;6:313–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

