Novel mouse hemostasis model for real-time determination of bleeding time and hemostatic plug composition
- PMID: 25442192
- PMCID: PMC4414118
- DOI: 10.1111/jth.12802
Novel mouse hemostasis model for real-time determination of bleeding time and hemostatic plug composition
Abstract
Introduction: Hemostasis is a rapid response by the body to stop bleeding at sites of vessel injury. Both platelets and fibrin are important for the formation of a hemostatic plug. Mice have been used to uncover the molecular mechanisms that regulate the activation of platelets and coagulation under physiologic conditions. However, measurements of hemostasis in mice are quite variable, and current methods do not quantify platelet adhesion or fibrin formation at the site of injury.
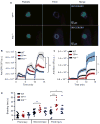
Methods: We describe a novel hemostasis model that uses intravital fluorescence microscopy to quantify platelet adhesion, fibrin formation and time to hemostatic plug formation in real time. Repeated vessel injuries of ~ 50-100 μm in diameter were induced with laser ablation technology in the saphenous vein of mice.
Results: Hemostasis in this model was strongly impaired in mice deficient in glycoprotein Ibα or talin-1, which are important regulators of platelet adhesiveness. In contrast, the time to hemostatic plug formation was only minimally affected in mice deficient in the extrinsic tissue factor (TF(low)) or the intrinsic factor IX coagulation pathways, even though platelet adhesion was significantly reduced. A partial reduction in platelet adhesiveness obtained with clopidogrel led to instability within the hemostatic plug, especially when combined with impaired coagulation in TF(low) mice.
Conclusions: In summary, we present a novel, highly sensitive method to quantify hemostatic plug formation in mice. On the basis of its sensitivity to platelet adhesion defects and its real-time imaging capability, we propose this model as an ideal tool with which to study the efficacy and safety of antiplatelet agents.
Keywords: blood coagulation; blood platelets; fluorescence imaging; hemostasis; mice.
© 2014 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
The authors state that they have no conflict of interest.
Figures



Comment in
-
Down in a hole: a new laser ablation model of hemostasis.J Thromb Haemost. 2015 Mar;13(3):414-6. doi: 10.1111/jth.12820. Epub 2015 Jan 9. J Thromb Haemost. 2015. PMID: 25510738 No abstract available.
References
-
- Broos K, Feys HB, De Meyer SF, Vanhoorelbeke K, Deckmyn H. Platelets at work in primary hemostasis. Blood Rev. 2011;25:155–67. - PubMed
-
- Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate–receptor interactions in platelet thrombus formation under flow. Cell. 1998;94:657–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

