Computational psychiatry
- PMID: 25442941
- PMCID: PMC4255477
- DOI: 10.1016/j.neuron.2014.10.018
Computational psychiatry
Abstract
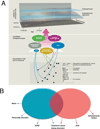
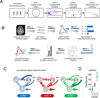
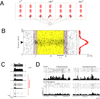
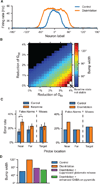
Psychiatric disorders such as autism and schizophrenia, arise from abnormalities in brain systems that underlie cognitive, emotional, and social functions. The brain is enormously complex and its abundant feedback loops on multiple scales preclude intuitive explication of circuit functions. In close interplay with experiments, theory and computational modeling are essential for understanding how, precisely, neural circuits generate flexible behaviors and their impairments give rise to psychiatric symptoms. This Perspective highlights recent progress in applying computational neuroscience to the study of mental disorders. We outline basic approaches, including identification of core deficits that cut across disease categories, biologically realistic modeling bridging cellular and synaptic mechanisms with behavior, and model-aided diagnosis. The need for new research strategies in psychiatry is urgent. Computational psychiatry potentially provides powerful tools for elucidating pathophysiology that may inform both diagnosis and treatment. To achieve this promise will require investment in cross-disciplinary training and research in this nascent field.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

