Syngap1 haploinsufficiency damages a postnatal critical period of pyramidal cell structural maturation linked to cortical circuit assembly
- PMID: 25444158
- PMCID: PMC4326604
- DOI: 10.1016/j.biopsych.2014.08.001
Syngap1 haploinsufficiency damages a postnatal critical period of pyramidal cell structural maturation linked to cortical circuit assembly
Abstract
Background: Genetic haploinsufficiency of SYNGAP1/Syngap1 commonly occurs in developmental brain disorders, such as intellectual disability, epilepsy, schizophrenia, and autism spectrum disorder. Thus, studying mouse models of Syngap1 haploinsufficiency may uncover pathologic developmental processes common among distinct brain disorders.
Methods: A Syngap1 haploinsufficiency model was used to explore the relationship between critical period dendritic spine abnormalities, cortical circuit assembly, and the window for genetic rescue to understand how damaging mutations disrupt key substrates of mouse brain development.
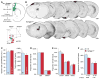
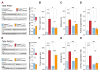
Results: Syngap1 mutations broadly disrupted a developmentally sensitive period that corresponded to the period of heightened postnatal cortical synaptogenesis. Pathogenic Syngap1 mutations caused a coordinated acceleration of dendrite elongation and spine morphogenesis and pruning of these structures in neonatal cortical pyramidal neurons. These mutations also prevented a form of developmental structural plasticity associated with experience-dependent reorganization of brain circuits. Consistent with these findings, Syngap1 mutant mice displayed an altered pattern of long-distance synaptic inputs into a cortical area important for cognition. Interestingly, the ability to genetically improve the behavioral endophenotype of Syngap1 mice decreased slowly over postnatal development and mapped onto the developmental period of coordinated dendritic insults.
Conclusions: Pathogenic Syngap1 mutations have a profound impact on the dynamics and structural integrity of pyramidal cell postsynaptic structures known to guide the de novo wiring of nascent cortical circuits. These findings support the idea that disrupted critical periods of dendritic growth and spine plasticity may be a common pathologic process in developmental brain disorders.
Keywords: Autism spectrum disorder; Development; Epilepsy; Intellectual disability; Mouse model; Synapse; Syngap1.
Copyright © 2015 Society of Biological Psychiatry. All rights reserved.
Figures




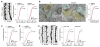

References
-
- de Ligt J, Willemsen MH, van Bon BW, Kleefstra T, Yntema HG, Kroes T, et al. Diagnostic exome sequencing in persons with severe intellectual disability. The New England journal of medicine. 2012;367:1921–1929. - PubMed
-
- Rauch A, Wieczorek D, Graf E, Wieland T, Endele S, Schwarzmayr T, et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet. 2012;380:1674–1682. - PubMed
-
- Stefansson H, Meyer-Lindenberg A, Steinberg S, Magnusdottir B, Morgen K, Arnarsdottir S, et al. CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature. 2014;505:361–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

