Ganetespib, a novel Hsp90 inhibitor in patients with KRAS mutated and wild type, refractory metastatic colorectal cancer
- PMID: 25444464
- PMCID: PMC5489410
- DOI: 10.1016/j.clcc.2014.09.001
Ganetespib, a novel Hsp90 inhibitor in patients with KRAS mutated and wild type, refractory metastatic colorectal cancer
Abstract
Background: Heat shock protein 90 (Hsp90) is a cellular chaperone that is required for the maturation and stability of a variety of proteins that play key roles in colon cancer initiation and progression. The primary objective of the current study was to define the safety and efficacy of ganetespib, a novel, selective small-molecule Hsp90 inhibitor, in patients with refractory metastatic colorectal cancer.
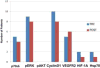
Patients and methods: The study was a single-arm, Simon 2-stage, phase II trial for patients with chemotherapy-refractory, metastatic colorectal cancer. Patients received ganetespib 200 mg/m(2) intravenously. Tumor tissue was collected before treatment and 48 hours after treatment for changes in expression of Hsp90 client proteins and other potential pharmacodynamics markers. V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS), v-Raf murine sarcoma viral oncogene homolog B, and phosphatidylinositol-4, 5-bisphosphate 3-kinase, catalytic subunit alpha (PIK3CA) mutational status was also determined.
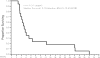
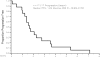
Results: Seventeen patients were treated (median age, 58; range, 44-79 years). No patients demonstrated objective regression of disease. Two patients had stable disease of 6.8 and 5.1 months duration. Serious adverse events that were potentially attributable to ganetespib included diarrhea (12%, n = 2), fatigue (17%, n = 3), and increased aspartate aminotransferase/alanine aminotransferase (12%, n = 2) and alkaline phosphatase (6%, n = 1) levels. Of the 17 evaluable patients, 9 (53%) including patients with stable disease as best response, had KRAS-mutant tumors.
Conclusion: In this first phase II investigation of an Hsp90 inhibitor in colorectal cancer, ganetespib as a single agent did not demonstrate activity in chemotherapy-refractory metastatic colorectal cancer. However, on the basis of the drug's promising preclinical combination data and the relatively mild toxicity profile, further clinical investigation of this agent in combination with standard cytotoxic agents is planned.
Keywords: Ganetespib; HSP 90; KRAS; Metastatic colorectal cancer; Single agent.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Dr. Proia is an employee of Synta Pharmaceuticals. All other authors declare no conflicts of interest.
Figures
References
-
- Protti MP, Heltai S, Bellone M, et al. Constitutive expression of the heat shock protein 72 kDa in human melanoma cells. Cancer Lett. 1994;85:211–6. - PubMed
-
- Mileo AM, Fanuele M, Battaglia F, et al. Selective over-expression of mRNA coding for 90 KDa stress-protein in human ovarian cancer. Anticancer Res. 1990;10:903–6. - PubMed
-
- Ehrenfried JA, Herron BE, Townsend CM, Jr, et al. Heat shock proteins are differentially expressed in human gastrointestinal cancers. Surg Oncol. 1995;4:197–203. - PubMed
-
- Pratt WB. The hsp90-based chaperone system: involvement in signal transduction from a variety of hormone and growth factor receptors. Proc Soc Exp Biol Med. 1998;217:420–34. - PubMed
-
- Whitesell L, Bagatell R, Falsey R. The stress response: implications for the clinical development of hsp90 inhibitors. Curr Cancer Drug Targets. 2003;3:349–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

