Chemokines in tuberculosis: the good, the bad and the ugly
- PMID: 25444549
- PMCID: PMC4314384
- DOI: 10.1016/j.smim.2014.09.004
Chemokines in tuberculosis: the good, the bad and the ugly
Abstract
Mycobacterium tuberculosis (Mtb) infects about one-third of the world's population, with a majority of infected individuals exhibiting latent asymptomatic infection, while 5-10% of infected individuals progress to active pulmonary disease. Research in the past two decades has elucidated critical host immune mechanisms that mediate Mtb control. Among these, chemokines have been associated with numerous key processes that lead to Mtb containment, from recruitment of myeloid cells into the lung to activation of adaptive immunity, formation of protective granulomas and vaccine recall responses. However, imbalances in several key chemokine mediators can alter the delicate balance of cytokines and cellular responses that promote mycobacterial containment, instead precipitating terminal tissue destruction and spread of Mtb infection. In this review, we will describe recent insights in the involvement of chemokines in host responses to Mtb infection and Mtb containment (the good), chemokines contributing to inflammation during TB (the bad), and the role of chemokines in driving cavitation and lung pathology (the ugly).
Keywords: Chemokines; Lung; Mycobacterial infections.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

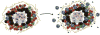
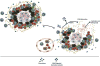
References
-
- Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for tuberculosis elimination. Annual review of public health. 2013;34:271–286. - PubMed
-
- Zlotnik A, Yoshie O. Chemokines : A New Classification System and Their Role in Immunity. Immunity. 2000;12:121–127. - PubMed
-
- Flynn JL. Lessons from experimental Mycobacterium tuberculosis infections. Microbes Infect. 2006;8:1179–1188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

