Phase II trial in adults of concurrent or sequential 2009 pandemic H1N1 and 2009-2010 seasonal trivalent influenza vaccinations
- PMID: 25444805
- PMCID: PMC4293632
- DOI: 10.1016/j.vaccine.2014.10.083
Phase II trial in adults of concurrent or sequential 2009 pandemic H1N1 and 2009-2010 seasonal trivalent influenza vaccinations
Abstract
Background: During the 2009 influenza pandemic both seasonal and 2009 pandemic vaccines were recommended. We conducted a randomized trial of monovalent 2009-H1N1 vaccine and seasonal trivalent inactivated influenza vaccine (IIV3) given sequentially or concurrently to adults.
Methods: Adults randomized to 4 study groups and stratified by age (18-64 and ≥65 years) received 1 dose of seasonal IIV3 or placebo and 2 doses of 2009-H1N1 vaccine or placebo in one of 4 combinations, i.e., H1N1+Placebo/H1N1+Placebo/IIV3 (HP/HP/V3), H1N1+IIV3/H1N1+Placebo/Placebo (HV3/HP/P), H1N1+Placebo/H1N1+IIV3/Placebo (HP/HV3/P), and IIV3+Placebo/H1N1+Placebo/H1N1 (V3P/HP/H). Intramuscular injections were given three times at 21 day intervals. Sera for antibody assays were obtained prior to and 21 days after each vaccination. Reactogenicity and adverse events were monitored.
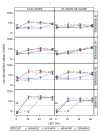
Results: Eight hundred-five (805) adults were enrolled. All combinations of vaccines were safe and well tolerated. In general, one dose of 2009-H1N1 and one dose of IIV3, regardless of sequence or concurrency of administration, were immunogenic in adults. There were no significant differences in geometric mean titers (GMT) or the proportions of subjects with ≥4-fold rise in antibody responses and titers ≥40 for any vaccine group or between age strata for 2009-H1N1 after the first or second dose, although the vaccine sequence affected the titers to the IIV3 antigens. Hemagglutination inhibition antibody (HAI) GMTs against 2009-H1N1 for the combined age strata 21 days after the first 2009-H1N1 dose were 190.4, 182.1, 232.9 and 157.5 for HP/HP/V3, HV3/HP/P, HP/HV3/P and V3P/HP/H, respectively. While IIV3 GMTs were adequate they were generally lower than the 2009-H1N1 GMTs. In a subset of subjects, there was good correlation between HAI and microneutralization (MN) titers (Spearman's correlation coefficient 0.92).
Conclusions: All vaccine combinations were generally well tolerated. Immune responses to one dose of 2009-H1N1 were adequate regardless of the sequence of vaccination in all age groups, but the sequence affected titers to IIV3 antigens.
Keywords: 2009-H1N1; Adults; Concurrent; Elderly; HAI; Influenza vaccine; Microneutralization; Pandemic; Seasonal IIV3; Sequential.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Sharon Frey, David Bernstein, Wendy Keitel, Hana El Sahly, Rebecca Brady, Nadine Georges Rouphael, Mark J. Mulligan, Heather Hill, Mark Wolff, Diana Noah, Robert Belshe, Robert L. Atmar, Srilatha Edupuganti, Shital M. Patel, Irene Graham and Edwin Anderson have no conflicts of interest to declare.
Figures

Similar articles
-
Safety and immune responses in children after concurrent or sequential 2009 H1N1 and 2009-2010 seasonal trivalent influenza vaccinations.J Infect Dis. 2012 Sep 15;206(6):828-37. doi: 10.1093/infdis/jis445. Epub 2012 Jul 16. J Infect Dis. 2012. PMID: 22802432 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of high-dose trivalent inactivated influenza vaccine in adults 50-64 years of age.Vaccine. 2015 Dec 16;33(51):7188-7193. doi: 10.1016/j.vaccine.2015.10.131. Epub 2015 Nov 7. Vaccine. 2015. PMID: 26555348 Clinical Trial.
-
Safety and immunogenicity of a virus-like particle pandemic influenza A (H1N1) 2009 vaccine in a blinded, randomized, placebo-controlled trial of adults in Mexico.Vaccine. 2011 Oct 13;29(44):7826-34. doi: 10.1016/j.vaccine.2011.07.099. Epub 2011 Aug 2. Vaccine. 2011. PMID: 21816199 Free PMC article. Clinical Trial.
-
Immunogenicity of high-dose trivalent inactivated influenza vaccine: a systematic review and meta-analysis.Expert Rev Vaccines. 2019 Mar;18(3):295-308. doi: 10.1080/14760584.2019.1575734. Epub 2019 Feb 13. Expert Rev Vaccines. 2019. PMID: 30689467
-
Adult Vaccine Coadministration Is Safe, Effective, and Acceptable: Results of a Survey of the Literature.Influenza Other Respir Viruses. 2025 Mar;19(3):e70090. doi: 10.1111/irv.70090. Influenza Other Respir Viruses. 2025. PMID: 40045565 Free PMC article. Review.
Cited by
-
Refining the approach to vaccines against influenza A viruses with pandemic potential.Future Virol. 2015;10(9):1033-1047. doi: 10.2217/fvl.15.69. Future Virol. 2015. PMID: 26587050 Free PMC article.
-
Racial and Ethnic Diversity in SARS-CoV-2 Vaccine Clinical Trials Conducted in the United States.Vaccines (Basel). 2022 Feb 14;10(2):290. doi: 10.3390/vaccines10020290. Vaccines (Basel). 2022. PMID: 35214748 Free PMC article. Review.
-
Systems vaccinology: Enabling rational vaccine design with systems biological approaches.Vaccine. 2015 Sep 29;33(40):5294-301. doi: 10.1016/j.vaccine.2015.03.072. Epub 2015 Apr 6. Vaccine. 2015. PMID: 25858860 Free PMC article. Review.
References
-
- Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. Erratum, N Engl J Med 2009;361:1027. - PubMed
-
- Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April – June 2009. N Engl J Med. 2009;361:1935–44. - PubMed
-
- France AM, Jackson M, Schrag S, et al. Household transmission of 2009 influenza A (H1N1) virus after a school-based outbreak in New York City, April-May 2009. J Infect Dis. 2010;201:984–92. - PubMed
-
- Centers for Disease control and Prevention. Update: Novel influenza A (H1N1) virus infections – worldwide, May 6, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:453–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

