Autologous aldrithiol-2-inactivated HIV-1 combined with polyinosinic-polycytidylic acid-poly-L-lysine carboxymethylcellulose as a vaccine platform for therapeutic dendritic cell immunotherapy
- PMID: 25444812
- PMCID: PMC4272884
- DOI: 10.1016/j.vaccine.2014.10.054
Autologous aldrithiol-2-inactivated HIV-1 combined with polyinosinic-polycytidylic acid-poly-L-lysine carboxymethylcellulose as a vaccine platform for therapeutic dendritic cell immunotherapy
Abstract
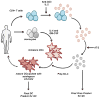
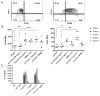
Therapeutic interventions for HIV-1 that successfully augment adaptive immunity to promote killing of infected cells may be a requisite component of strategies to reduce latent cellular reservoirs. Adoptive immunotherapies utilizing autologous monocyte-derived dendritic cells (DCs) that have been activated and antigen loaded ex vivo may serve to circumvent defects in DC function that are present during HIV infection in order to enhance adaptive immune responses. Here we detail the clinical preparation of DCs loaded with autologous aldrithiol-2 (AT-2)-inactivated HIV that have been potently activated with the viral mimic, Polyinosinic-polycytidylic acid-poly-l-lysine carboxymethylcellulose (Poly-ICLC). HIV is first propagated from CD4+ T cells from HIV-infected donors and then rendered non-replicative by chemical inactivation with aldrithiol-2 (AT-2), purified, and quantified. Viral inactivation is confirmed through measurement of Tat-regulated β-galactosidase reporter gene expression following infection of TZM-bl cells. In-process testing for sterility, mycoplasma, LPS, adventitious agents, and removal of AT-2 is performed on viral preparations. Autologous DCs are generated and pulsed with autologous AT-2-inactivated virus and simultaneously stimulated with Poly-ICLC to constitute the final DC vaccine product. Phenotypic identity, maturation, and induction of HIV-specific adaptive immune responses are confirmed via flow cytometric analysis of DCs and cocultured autologous CD4+ and CD8+ T cells. Lot release criteria for the DC vaccine have been defined in accordance with Good Manufacturing Practice (GMP) guidelines. The demonstrated feasibility of this approach has resulted in approval by the FDA for investigational use in antiretroviral (ART) suppressed individuals. We discuss how this optimized DC formulation may enhance the quality of anti-HIV adaptive responses beyond what has been previously observed during DC immunotherapy trials for HIV infection.
Keywords: Dendritic cell; HIV-1; Poly-ICLC; Therapeutic vaccine.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Nina Bhardwaj reports a potential conflict as a co-inventor on patents related to DC function and differentiation. Andres Salazar is the CEO of Oncovir, Inc which manufactures Poly-ICLC. The remaining authors report no conflicts.
Figures



Similar articles
-
Superiority in Rhesus Macaques of Targeting HIV-1 Env gp140 to CD40 versus LOX-1 in Combination with Replication-Competent NYVAC-KC for Induction of Env-Specific Antibody and T Cell Responses.J Virol. 2017 Apr 13;91(9):e01596-16. doi: 10.1128/JVI.01596-16. Print 2017 May 1. J Virol. 2017. PMID: 28202751 Free PMC article.
-
Therapeutic dendritic-cell vaccine for chronic HIV-1 infection.Nat Med. 2004 Dec;10(12):1359-65. doi: 10.1038/nm1147. Epub 2004 Nov 28. Nat Med. 2004. PMID: 15568033
-
Preparation of tumor antigen-loaded mature dendritic cells for immunotherapy.J Vis Exp. 2013 Aug 1;(78):50085. doi: 10.3791/50085. J Vis Exp. 2013. PMID: 23928481 Free PMC article.
-
The Evolution of Dendritic Cell Immunotherapy against HIV-1 Infection: Improvements and Outlook.J Immunol Res. 2020 May 25;2020:9470102. doi: 10.1155/2020/9470102. eCollection 2020. J Immunol Res. 2020. PMID: 32537473 Free PMC article. Review.
-
Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity.J Intern Med. 2012 Feb;271(2):183-92. doi: 10.1111/j.1365-2796.2011.02496.x. Epub 2012 Jan 4. J Intern Med. 2012. PMID: 22126373 Free PMC article. Review.
Cited by
-
Poly-ICLC, a TLR3 Agonist, Induces Transient Innate Immune Responses in Patients With Treated HIV-Infection: A Randomized Double-Blinded Placebo Controlled Trial.Front Immunol. 2019 Apr 9;10:725. doi: 10.3389/fimmu.2019.00725. eCollection 2019. Front Immunol. 2019. PMID: 31024557 Free PMC article. Clinical Trial.
-
Concurrent Exposure of Neutralizing and Non-neutralizing Epitopes on a Single HIV-1 Envelope Structure.Front Immunol. 2019 Jul 5;10:1512. doi: 10.3389/fimmu.2019.01512. eCollection 2019. Front Immunol. 2019. PMID: 31338095 Free PMC article.
-
Antiviral Cell Products against COVID-19: Learning Lessons from Previous Research in Anti-Infective Cell-Based Agents.Biomedicines. 2022 Apr 7;10(4):868. doi: 10.3390/biomedicines10040868. Biomedicines. 2022. PMID: 35453618 Free PMC article. Review.
-
Highly Efficient Autologous HIV-1 Isolation by Coculturing Macrophage With Enriched CD4+ T Cells From HIV-1 Patients.Front Virol. 2022 Apr;2:869431. doi: 10.3389/fviro.2022.869431. Epub 2022 Apr 7. Front Virol. 2022. PMID: 35967461 Free PMC article.
References
-
- Buisson S, et al. Monocyte-derived dendritic cells from HIV type 1-infected individuals show reduced ability to stimulate T cells and have altered production of interleukin (IL)-12 and IL-10. The Journal of infectious diseases. 2009;199(12):1862–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

