Neuromuscular nicotinic receptors mediate bladder contractions following bladder reinnervation with somatic to autonomic nerve transfer after decentralization by spinal root transection
- PMID: 25444958
- PMCID: PMC4400216
- DOI: 10.1016/j.juro.2014.10.046
Neuromuscular nicotinic receptors mediate bladder contractions following bladder reinnervation with somatic to autonomic nerve transfer after decentralization by spinal root transection
Abstract
Purpose: We investigated whether the reinnervated neuronal pathway mediates contraction via the same neurotransmitter and receptor mechanisms as the original pathway.
Materials and methods: After decentralizing the bladder by transecting the sacral roots in dogs we performed peripheral nerve transfer, including bilateral genitofemoral to pelvic nerve transfer and unilateral left femoral nerve to bilateral pelvic nerve transfer. Reinnervation was assessed 7.5 months postoperatively by monitoring bladder pressure during electrical stimulation of the transferred nerves, spinal ventral roots and spinal cord.
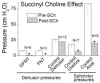
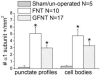
Results: Of the 17 dogs with genitofemoral to pelvic nerve transfer 14 (82%) demonstrated functional bladder reinnervation as evidenced by increased bladder pressure during stimulation of the transferred genitofemoral nerve, or L3 or L4 spinal ventral roots. Lumbar spinal cord stimulation caused increased bladder pressure in 9 of 10 dogs (90%) with unilateral left femoral nerve to bilateral pelvic nerve transfer. Succinylcholine virtually eliminated the bladder pressure increases induced by electrical stimulation of the transferred somatic nerves or of the lumbar spinal segments that contribute axons to these donor nerves. In unoperated or sham operated controls succinylcholine had no effect on nerve evoked bladder pressure increases but it substantially decreased the urethral and anal sphincter pressure induced by stimulating the lumbosacral spinal cord or the S2-S3 spinal ventral roots. The reinnervated detrusor muscles of dogs with genitofemoral to pelvic nerve transfer and unilateral left femoral nerve to bilateral pelvic nerve transfer also showed increased α1 nicotinic receptor subunit immunoreactivity in punctate dots on detrusor muscle fascicles and in neuronal cell bodies. This staining was not observed in controls.
Conclusions: Succinylcholine sensitive nicotinic receptors, which normally mediate only skeletal muscle neuromuscular junction neurotransmission, appeared in the new neuronal pathway after genitofemoral to pelvic and unilateral femoral nerve to bilateral pelvic nerve transfer. This suggests end organ neuroplasticity after reinnervation by somatic motor axons.
Keywords: nerve regeneration; nerve transfer; nicotinic; receptors; spinal cord injuries; urinary bladder.
Copyright © 2015 American Urological Association Education and Research, Inc. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Bladder reinnervation using a primarily motor donor nerve (femoral nerve branches) is functionally superior to using a primarily sensory donor nerve (genitofemoral nerve).J Urol. 2015 Mar;193(3):1042-51. doi: 10.1016/j.juro.2014.07.095. Epub 2014 Jul 24. J Urol. 2015. PMID: 25066874 Free PMC article.
-
Functional reinnervation of the canine bladder after spinal root transection and immediate somatic nerve transfer.J Neurotrauma. 2008 Mar;25(3):214-24. doi: 10.1089/neu.2007.0328. J Neurotrauma. 2008. PMID: 18352835 Free PMC article.
-
Bladder reinnervation by somatic nerve transfer to pelvic nerve vesical branches does not reinnervate the urethra.Neurourol Urodyn. 2020 Jan;39(1):181-189. doi: 10.1002/nau.24217. Epub 2019 Nov 13. Neurourol Urodyn. 2020. PMID: 31724210 Free PMC article.
-
Neural reconstruction methods of restoring bladder function.Nat Rev Urol. 2015 Feb;12(2):100-18. doi: 10.1038/nrurol.2015.4. Nat Rev Urol. 2015. PMID: 25666987 Free PMC article. Review.
-
[Bladder reinnervation with creation of a "somato-autonomic" reflex pathway in spinal cord injured or spina bifida, a new way for treatment?].Prog Urol. 2011 Sep;21(8):501-7. doi: 10.1016/j.purol.2011.04.002. Epub 2011 May 14. Prog Urol. 2011. PMID: 21872150 Review. French.
Cited by
-
Nerve transfer for restoration of lower motor neuron-lesioned bladder, urethral and anal sphincter function. Part 4: Effectiveness of the motor reinnervation.Am J Physiol Regul Integr Comp Physiol. 2024 Jun 1;326(6):R528-R551. doi: 10.1152/ajpregu.00248.2023. Epub 2024 Mar 18. Am J Physiol Regul Integr Comp Physiol. 2024. PMID: 38497126 Free PMC article.
-
Dog and human bladders have different neurogenic and nicotinic responses in inner versus outer detrusor muscle layers.Am J Physiol Regul Integr Comp Physiol. 2022 Oct 1;323(4):R589-R600. doi: 10.1152/ajpregu.00084.2022. Epub 2022 Sep 5. Am J Physiol Regul Integr Comp Physiol. 2022. PMID: 36062901 Free PMC article.
-
Peripheral nerve transfers for dysfunctions in central nervous system injuries: a systematic review.Int J Surg. 2024 Jun 1;110(6):3814-3826. doi: 10.1097/JS9.0000000000001267. Int J Surg. 2024. PMID: 38935818 Free PMC article.
-
Translational Challenges of Rat Models of Upper Extremity Dysfunction After Spinal Cord Injury.Top Spinal Cord Inj Rehabil. 2018 Summer;24(3):195-205. doi: 10.1310/sci2403-195. Top Spinal Cord Inj Rehabil. 2018. PMID: 29997423 Free PMC article. Review.
-
Current Progress on Central Cholinergic Receptors as Therapeutic Targets for Alzheimer's Disease.Curr Alzheimer Res. 2024;21(1):50-68. doi: 10.2174/0115672050306008240321034006. Curr Alzheimer Res. 2024. PMID: 38529600 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

