Cdk1, PKCδ and calcineurin-mediated Drp1 pathway contributes to mitochondrial fission-induced cardiomyocyte death
- PMID: 25445585
- PMCID: PMC4312217
- DOI: 10.1016/j.bbrc.2014.09.144
Cdk1, PKCδ and calcineurin-mediated Drp1 pathway contributes to mitochondrial fission-induced cardiomyocyte death
Abstract
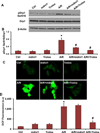
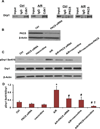
Myocardial ischemia-reperfusion (I/R) injury is one of the leading causes of death and disability worldwide. Mitochondrial fission has been shown to be involved in cardiomyocyte death. However, molecular machinery involved in mitochondrial fission during I/R injury has not yet been completely understood. In this study we aimed to investigate molecular mechanisms of controlling activation of dynamin-related protein 1 (Drp1, a key protein in mitochondrial fission) during anoxia-reoxygenation (A/R) injury of HL1 cardiomyocytes. A/R injury induced cardiomyocyte death accompanied by the increases of mitochondrial fission, reactive oxygen species (ROS) production and activated Drp1 (pSer616 Drp1), and decrease of inactivated Drp1 (pSer637 Drp1) while mitochondrial fusion protein levels were not significantly changed. Blocking Drp1 activity with mitochondrial division inhibitor mdivi1 attenuated cell death, mitochondrial fission, and Drp1 activation after A/R. Trolox, a ROS scavenger, decreased pSer616 Drp1 level and mitochondrial fission after A/R. Immunoprecipitation assay further indicates that cyclin dependent kinase 1 (Cdk1) and protein kinase C isoform delta (PKCδ) bind Drp1, thus increasing mitochondrial fission. Inhibiting Cdk1 and PKCδ attenuated the increases in pSer616 Drp1, mitochondrial fission, and cardiomyocyte death. FK506, a calcineurin inhibitor, blocked the decrease in expression of inactivated pSer637 Drp1 and mitochondrial fission. Our findings reveal the following novel molecular mechanisms controlling mitochondrial fission during A/R injury of cardiomyocytes: (1) ROS are upstream initiators of mitochondrial fission; and (2) the increased mitochondrial fission is resulted from both increased activation and decreased inactivation of Drp1 through Cdk1, PKCδ, and calcineurin-mediated pathways, respectively.
Keywords: Cardiomyocyte; Ischemia–reperfusion injury; Mitochondrial dynamics; Reactive oxygen species.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures








References
-
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. - PubMed
-
- Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans. 2006;34:232–237. - PubMed
-
- Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. - PubMed
-
- Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

