Novel mechanisms for the vitamin D receptor (VDR) in the skin and in skin cancer
- PMID: 25445917
- PMCID: PMC4361259
- DOI: 10.1016/j.jsbmb.2014.10.017
Novel mechanisms for the vitamin D receptor (VDR) in the skin and in skin cancer
Abstract
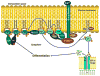
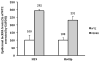
The VDR acting with or without its principal ligand 1,25(OH)2D regulates two central processes in the skin, interfollicular epidermal (IFE) differentiation and hair follicle cycling (HFC). Calcium is an important co-regulator with 1,25(OH)2D at least of epidermal differentiation. Knockout of the calcium sensing receptor (CaSR) in addition to VDR accelerates the development of skin cancer in mice on a low calcium diet. Coactivators such as mediator 1 (aka DRIP205) and steroid receptor coactivator 3 (SRC3) regulate VDR function at different stages of the differentiation process, with Med 1 essential for hair follicle differentiation and early stages of epidermal differentiation and proliferation and SRC3 essential for the latter stages of differentiation including formation of the permeability barrier and innate immunity. The corepressor of VDR, hairless (HR), is essential for hair follicle cycling, although its effect on epidermal differentiation in vivo is minimal. In its regulation of HFC and IFE VDR controls two pathways-wnt/β-catenin and sonic hedgehog (SHH). In the absence of VDR these pathways are overexpressed leading to tumor formation. Whereas, VDR binding to β-catenin may block its activation of TCF/LEF1 sites, β-catenin binding to VDR may enhance its activation of VDREs. 1,25(OH)2D promotes but may not be required for these interactions. Suppression of SHH expression by VDR, on the other hand, requires 1,25(OH)2D. The major point of emphasis is that the role of VDR in the skin involves a number of novel mechanisms, both 1,25(OH)2D dependent and independent, that when disrupted interfere with IFE differentiation and HFC, predisposing to cancer formation. This article is part of a Special Issue entitled '17th Vitamin D Workshop'.
Keywords: Calcium sensing receptor; Cancer; Coregulators; Epidermal differentiation; Hair follicle cycling; Vitamin D receptor.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
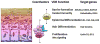

Similar articles
-
Reciprocal role of vitamin D receptor on β-catenin regulated keratinocyte proliferation and differentiation.J Steroid Biochem Mol Biol. 2014 Oct;144 Pt A:237-41. doi: 10.1016/j.jsbmb.2013.11.002. Epub 2013 Nov 12. J Steroid Biochem Mol Biol. 2014. PMID: 24239508 Free PMC article. Review.
-
1α,25(OH)2-dihydroxyvitamin D3/VDR protects the skin from UVB-induced tumor formation by interacting with the β-catenin pathway.J Steroid Biochem Mol Biol. 2013 Jul;136:229-32. doi: 10.1016/j.jsbmb.2012.09.024. Epub 2012 Sep 28. J Steroid Biochem Mol Biol. 2013. PMID: 23026511 Free PMC article. Review.
-
The vitamin D receptor: a tumor suppressor in skin.Discov Med. 2011 Jan;11(56):7-17. Discov Med. 2011. PMID: 21276406 Free PMC article.
-
Sequential regulation of keratinocyte differentiation by 1,25(OH)2D3, VDR, and its coregulators.J Steroid Biochem Mol Biol. 2007 Mar;103(3-5):396-404. doi: 10.1016/j.jsbmb.2006.12.063. Epub 2007 Jan 16. J Steroid Biochem Mol Biol. 2007. PMID: 17229570
-
Vitamin D and its receptor during late development.Biochim Biophys Acta. 2015 Feb;1849(2):171-80. doi: 10.1016/j.bbagrm.2014.05.026. Epub 2014 Jun 3. Biochim Biophys Acta. 2015. PMID: 24939836 Review.
Cited by
-
The CaSR Modulator NPS-2143 Reduced UV-Induced DNA Damage in Skh:hr1 Hairless Mice but Minimally Inhibited Skin Tumours.Int J Mol Sci. 2023 Mar 3;24(5):4921. doi: 10.3390/ijms24054921. Int J Mol Sci. 2023. PMID: 36902353 Free PMC article.
-
Decreased progenitor TCF1 + T-cells correlate with COVID-19 disease severity.Commun Biol. 2024 May 3;7(1):526. doi: 10.1038/s42003-024-05922-2. Commun Biol. 2024. PMID: 38702425 Free PMC article.
-
Anti-aging Strategies and Topical Delivery of Biopolymer-based Nanocarriers for Skin Cancer Treatment.Curr Aging Sci. 2024;17(1):31-48. doi: 10.2174/1874609816666230320122018. Curr Aging Sci. 2024. PMID: 36941817 Review.
-
Transcriptomic Response to 1,25-Dihydroxyvitamin D in Human Fibroblasts with or without a Functional Vitamin D Receptor (VDR): Novel Target Genes and Insights into VDR Basal Transcriptional Activity.Cells. 2019 Apr 5;8(4):318. doi: 10.3390/cells8040318. Cells. 2019. PMID: 30959822 Free PMC article.
-
Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions.Endocr Rev. 2019 Aug 1;40(4):1109-1151. doi: 10.1210/er.2018-00126. Endocr Rev. 2019. PMID: 30321335 Free PMC article. Review.
References
-
- Bikle DD, Pillai S. Vitamin D, calcium, and epidermal differentiation. Endocrine Review. 1993;14:3–19. - PubMed
-
- Bikle DD, Elalieh H, Chang S, Xie Z, Sundberg JP. Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol. 2006;207(2):340–53. - PubMed
-
- Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Choi EH, Dardenne O, Xie Z, Arnaud RS, Feingold K, Elias PM. 25 Hydroxyvitamin D 1 alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol. 2004;122(4):984–92. - PubMed
-
- Menon GK, Grayson S, Elias PM. Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion-capture cytochemistry. Journal of Investigative Dermatology. 1985;84(6):508–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

