Neurogranin binds α-synuclein in the human superior temporal cortex and interaction is decreased in Parkinson's disease
- PMID: 25446004
- PMCID: PMC4943923
- DOI: 10.1016/j.brainres.2014.10.013
Neurogranin binds α-synuclein in the human superior temporal cortex and interaction is decreased in Parkinson's disease
Erratum in
-
Corrigendum to "Neurogranin binds alpha-synuclein in the human superior temporal cortex and interaction is decreased in Parkinson's disease" [Brain Res. 1591 (2014) 102-110].Brain Res. 2025 Mar 15;1851:149504. doi: 10.1016/j.brainres.2025.149504. Epub 2025 Feb 13. Brain Res. 2025. PMID: 39947974 No abstract available.
Abstract
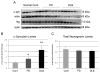
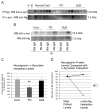
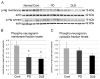
Neurogranin is a calmodulin binding protein that has been implicated in learning and memory, long-term potentiation and synaptic plasticity. Neurons expressing neurogranin in the cortex degenerate in late stages of Parkinson's disease with widespread α-synuclein pathology. While analyzing neurogranin gene expression levels through rtPCR in brains of mouse models overexpressing human α-synuclein, we found levels were elevated 2.5 times when compared to nontransgenic animals. Immunohistochemistry in the cortex revealed colocalization between α-synuclein and neurogranin in mouse transgenics when compared to control mice. Coimmunoprecipitation studies in the superior temporal cortex in humans confirmed interaction between α-synuclein and neurogranin, and decreased interaction between α-synuclein and neurogranin was noticed in patients diagnosed with Parkinson's disease when compared to normal control brains. Additionally, phosphorylated neurogranin levels were also decreased in the human superior temporal cortex in patients diagnosed with Parkinson's disease and patients diagnosed with dementia with Lewy bodies. Here, we show for the first time that neurogranin binds to α-synuclein in the human cortex, and this interaction decreases in Parkinson's disease along with the phosphorylation of neurogranin, a molecular process thought to be involved in learning and memory.
Keywords: Calmodulin; Dementia with Lewy bodies; LTP; Neurogranin; Parkinson’s disease; α-Synuclein.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures


References
-
- Alexander JA, et al. The diagnosis of dementia. J Arkansas Med Soc. 1987;83:365–368. - PubMed
-
- Baudier J, et al. Purification and characterization of a brain-specific protein kinase C substrate, neurogranin (p17). Identification of a consensus amino acid sequence between neurogranin and neuromodulin (GAP43) that corresponds to the protein kinase C phosphorylation site and the calmodulin-binding domain. J Biol Chem. 1991;266:229–237. - PubMed
-
- Chang JW, et al. Dendritic translocation of RC3/ neurogranin mRNA in normal aging, Alzheimer disease and frontotemporal dementia. J Neuropathol Exp Neurol. 1997;56:1105–1118. - PubMed
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

