Retinoic acid regulates Kit translation during spermatogonial differentiation in the mouse
- PMID: 25446031
- PMCID: PMC4268412
- DOI: 10.1016/j.ydbio.2014.10.020
Retinoic acid regulates Kit translation during spermatogonial differentiation in the mouse
Abstract
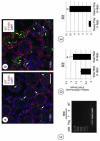
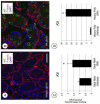
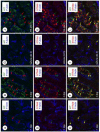
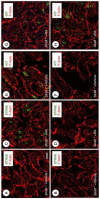
In the testis, a subset of spermatogonia retains stem cell potential, while others differentiate to eventually become spermatozoa. This delicate balance must be maintained, as defects can result in testicular cancer or infertility. Currently, little is known about the gene products and signaling pathways directing these critical cell fate decisions. Retinoic acid (RA) is a requisite driver of spermatogonial differentiation and entry into meiosis, yet the mechanisms activated downstream are undefined. Here, we determined a requirement for RA in the expression of KIT, a receptor tyrosine kinase essential for spermatogonial differentiation. We found that RA signaling utilized the PI3K/AKT/mTOR signaling pathway to induce the efficient translation of mRNAs for Kit, which are present but not translated in undifferentiated spermatogonia. Our findings provide an important molecular link between a morphogen (RA) and the expression of KIT protein, which together direct the differentiation of spermatogonia throughout the male reproductive lifespan.
Keywords: Kit; Retinoic acid; Spermatogenesis; Spermatogonia; Testis; Translation.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



References
-
- Arava Y. Isolation of polysomal RNA for microarray analysis. Methods Mol Biol. 2003;224:79–87. - PubMed
-
- Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet. 2006;38:1430–1434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

