Cyclic stretch of embryonic cardiomyocytes increases proliferation, growth, and expression while repressing Tgf-β signaling
- PMID: 25446186
- PMCID: PMC4302020
- DOI: 10.1016/j.yjmcc.2014.11.003
Cyclic stretch of embryonic cardiomyocytes increases proliferation, growth, and expression while repressing Tgf-β signaling
Abstract
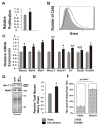
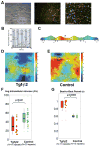
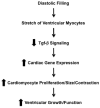
Perturbed biomechanical stimuli are thought to be critical for the pathogenesis of a number of congenital heart defects, including Hypoplastic Left Heart Syndrome (HLHS). While embryonic cardiomyocytes experience biomechanical stretch every heart beat, their molecular responses to biomechanical stimuli during heart development are poorly understood. We hypothesized that biomechanical stimuli activate specific signaling pathways that impact proliferation, gene expression and myocyte contraction. The objective of this study was to expose embryonic mouse cardiomyocytes (EMCM) to cyclic stretch and examine key molecular and phenotypic responses. Analysis of RNA-Sequencing data demonstrated that gene ontology groups associated with myofibril and cardiac development were significantly modulated. Stretch increased EMCM proliferation, size, cardiac gene expression, and myofibril protein levels. Stretch also repressed several components belonging to the Transforming Growth Factor-β (Tgf-β) signaling pathway. EMCMs undergoing cyclic stretch had decreased Tgf-β expression, protein levels, and signaling. Furthermore, treatment of EMCMs with a Tgf-β inhibitor resulted in increased EMCM size. Functionally, Tgf-β signaling repressed EMCM proliferation and contractile function, as assayed via dynamic monolayer force microscopy (DMFM). Taken together, these data support the hypothesis that biomechanical stimuli play a vital role in normal cardiac development and for cardiac pathology, including HLHS.
Keywords: Cardiac development; Cardiomyocytes; Contractility; Gene regulation; Hypoplastic Left Heart Syndrome; Mechanical stretch.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
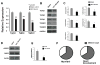

References
-
- Menon SC, Keenan HT, Weng HY, Lambert LM, Burch PT, Edwards R, et al. Outcome and resource utilization of infants born with hypoplastic left heart syndrome in the Intermountain West. The American journal of cardiology. 2012;110:720–7. - PubMed
-
- Dasgupta C, Martinez AM, Zuppan CW, Shah MM, Bailey LL, Fletcher WH. Identification of connexin43 (alpha1) gap junction gene mutations in patients with hypoplastic left heart syndrome by denaturing gradient gel electrophoresis (DGGE) Mutat Res. 2001;479:173–86. - PubMed
-
- Elliott DA, Kirk EP, Yeoh T, Chandar S, McKenzie F, Taylor P, et al. Cardiac homeobox gene NKX2–5 mutations and congenital heart disease: associations with atrial septal defect and hypoplastic left heart syndrome. J Am Coll Cardiol. 2003;41:2072–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

