Using c-fos to study neuronal ensembles in corticostriatal circuitry of addiction
- PMID: 25446457
- PMCID: PMC4427550
- DOI: 10.1016/j.brainres.2014.11.005
Using c-fos to study neuronal ensembles in corticostriatal circuitry of addiction
Abstract
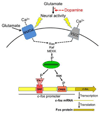
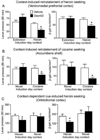
Learned associations between drugs and environment play an important role in addiction and are thought to be encoded within specific patterns of sparsely distributed neurons called neuronal ensembles. This hypothesis is supported by correlational data from in vivo electrophysiology and cellular imaging studies in relapse models in rodents. In particular, cellular imaging with the immediate early gene c-fos and its protein product Fos has been used to identify sparsely distributed neurons that were strongly activated during conditioned drug behaviors such as drug self-administration and context- and cue-induced reinstatement of drug seeking. Here we review how Fos and the c-fos promoter have been employed to demonstrate causal roles for Fos-expressing neuronal ensembles in prefrontal cortex and nucleus accumbens in conditioned drug behaviors. This work has allowed identification of unique molecular and electrophysiological alterations within Fos-expressing neuronal ensembles that may contribute to the development and expression of learned associations in addiction.
Keywords: Conditioned cues; Daun02 inactivation; Drug environment; Extinction; Nucleus accumbens; Prefrontal cortex; Self-administration.
Published by Elsevier B.V.
Figures

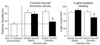
References
-
- Agell N, et al. Modulation of the Ras/Raf/MEK/ERK pathway by Ca(2+), and calmodulin. Cell Signal. 2002;14:649–654. - PubMed
-
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behavioral neuroscience. 1996;110:1397–1414. - PubMed
-
- Badiani A, et al. Environmental modulation of amphetamine-induced c-fos expression in D1 versus D2 striatal neurons. Behav Brain Res. 1999;103:203–209. - PubMed
-
- Badiani A, Robinson TE. Drug-induced neurobehavioral plasticity: the role of environmental context. Behavioural pharmacology. 2004;15:327–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

