Effects of the NOP agonist SCH221510 on producing and attenuating reinforcing effects as measured by drug self-administration in rats
- PMID: 25446568
- PMCID: PMC4259829
- DOI: 10.1016/j.ejphar.2014.10.029
Effects of the NOP agonist SCH221510 on producing and attenuating reinforcing effects as measured by drug self-administration in rats
Abstract
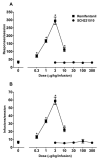
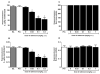
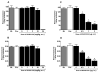
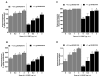
Nociceptin/orphanin FQ peptide (NOP) receptor agonists attenuate morphine-induced conditioned place preference in rodents. However, it is not known whether NOP agonists have reinforcing properties or can inhibit mu opioid receptor (MOP)-mediated reinforcement as measured by drug self-administration in rodents. Further understanding the behavioral effects of NOP agonists could suggest them as having potential in attenuating reinforcing effects of opioids. In the first part of the study, reinforcing properties of selective NOP agonist SCH221510 were determined and compared with the full MOP agonist remifentanil under fixed-ratio 5 (FR5) and progressive-ratio (PR) schedules of drug self-administration. In the second part, effects of systemic and intracisternal pretreatment of SCH221510 were determined and compared with MOP antagonist naltrexone in attenuating reinforcing effects of remifentanil and a non-drug reinforcer (sucrose pellets). Remifentanil self-administration (0.3-10 µg/kg/infusion) generated a biphasic dose-response curve, characteristic of drugs with reinforcing properties. SCH221510 (3-300 µg/kg/infusion) self-administration resulted in flat dose-response curves and early break-points under the PR, indicative of drugs lacking reinforcing value. Intracisternally, but not systemically, administered SCH221510 (0.3-3 µg) attenuated remifentanil self-administration, comparable with systemic naltrexone (0.03-0.3 mg/kg). SCH221510 (1-3 µg), unlike naltrexone (0.03-1 mg/kg), attenuated responding for sucrose pellets. Both effects of SCH221510 were reversed by the NOP antagonist J-113397 (0.3-3 µg). These results suggest that SCH221510 does not function as a reinforcer in rats, and that it can attenuate the reinforcing value of MOP agonists; therefore, the potential utility of NOP agonists for the treatment of drug addiction warrants further evaluation.
Keywords: Drug self-administration; NOP receptor; Nociceptin/orphanin FQ; Opioids; Remifentanil; SCH221510.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures


References
-
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. - PubMed
-
- Baylon GJ, Kaplan HL, Somer G, Busto UE, Sellers EM. Comparative abuse liability of intravenously administered remifentanil and fentanyl. J Clin Psychopharmacol. 2000;20:597–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

