Elevated levels of kynurenic acid during gestation produce neurochemical, morphological, and cognitive deficits in adulthood: implications for schizophrenia
- PMID: 25446576
- PMCID: PMC4731221
- DOI: 10.1016/j.neuropharm.2014.10.017
Elevated levels of kynurenic acid during gestation produce neurochemical, morphological, and cognitive deficits in adulthood: implications for schizophrenia
Abstract
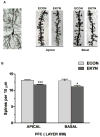
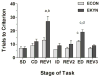
The levels of kynurenic acid (KYNA), an endogenous negative modulator of alpha7 nicotinic acetylcholine receptors (α7nAChRs), are elevated in the brains of patients with schizophrenia (SZ). We reported that increases of brain KYNA in rats, through dietary exposure to its precursor kynurenine from embryonic day (ED)15 to postnatal day (PD) 21, result in neurochemical and cognitive deficits in adulthood. The present experiments focused on the effects of prenatal exposure to elevated kynurenine on measures of prefrontal excitability known to be impaired in SZ. Pregnant dams were fed a mash containing kynurenine (100 mg/day; progeny = EKYNs) from ED15 until ED22. Controls were fed an unadulterated mash (progeny = ECONs). The dietary loading procedure elevated maternal and fetal plasma kynurenine (2223% and 693% above controls, respectively) and increased fetal KYNA (forebrain; 500% above controls) on ED21. Elevations in forebrain KYNA disappeared after termination of the loading (PD2), but KYNA levels in the prefrontal cortex (PFC) were unexpectedly increased again when measured in adults (PD56-80; 75% above controls). We also observed changes in several markers of prefrontal excitability, including expression of the α7nAChR (22% and 17% reductions at PD2 and PD56-80), expression of mGluR2 (31% and 24% reductions at ED21 and PD56-80), dendritic spine density (11-14% decrease at PD56-80), subsensitive mesolimbic stimulation of glutamate release in PFC, and reversal/extra-dimensional shift deficits in the prefrontally-mediated set-shifting task. These results highlight the deleterious impact of elevated KYNA levels during sensitive periods of early development, which model the pathophysiological and cognitive deficits seen in SZ.
Keywords: Alpha7 nicotinic receptors; Glutamate; Kynurenic acid; Prefrontal cortex; Schizophrenia; Set-shifting.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

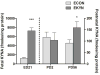
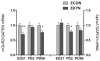

References
-
- Akbarian S, Bunney WE, Potkin SG, Wigal SB, Hagman JO, Sandman CA, Jones EG. Altered distribution of nicotinamide-adenine dinucleotide phosphate diaphorase cells in frontal-lobe of schizophrenics implies disturbances of cortical development. Archives of General Psychiatry. 1993;50:169–177. - PubMed
-
- Alexander KS, Bortz DM, Wu HQ, Brooks JM, Schwarcz R, Bruno JP. Perinatal elevations of kynurenic acid dysregulate prefrontal glutamate release and produce set-shifting deficits in adults: A new model of schizophrenia. Society for Neuroscience Meeting Planner 163.23. 2011 http://www.abstractsonline.com/plan/start.aspx?mkey=%7B8334BE29-8911-499....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

