The challenges and opportunities for the development of a T-cell epitope-based herpes simplex vaccine
- PMID: 25446827
- PMCID: PMC4254646
- DOI: 10.1016/j.vaccine.2014.10.002
The challenges and opportunities for the development of a T-cell epitope-based herpes simplex vaccine
Abstract
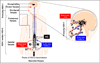
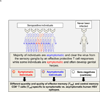
Herpes simplex virus type 1 and type 2 (HSV-1 & HSV-2) infections have been prevalent since the ancient Greek times. To this day, they still affect a staggering number of over a billion individuals worldwide. HSV-1 infections are predominant than HSV-2 infections and cause potentially blinding ocular herpes, oro-facial herpes and encephalitis. HSV-2 infections cause painful genital herpes, encephalitis, and death in newborns. While prophylactic and therapeutic HSV vaccines remain urgently needed for centuries, their development has been difficult. During the most recent National Institute of Health (NIH) workshop titled "Next Generation Herpes Simplex Virus Vaccines: The Challenges and Opportunities", basic researchers, funding agencies, and pharmaceutical representatives gathered: (i) to assess the status of herpes vaccine research; and (ii) to identify the gaps and propose alternative approaches in developing a safe and efficient herpes vaccine. One "common denominator" among previously failed clinical herpes vaccine trials is that they either used a whole virus or a whole viral protein, which contain both "pathogenic symptomatic" and "protective asymptomatic" antigens and epitopes. In this report, we continue to advocate developing "asymptomatic" epitope-based sub-unit vaccine strategies that selectively incorporate "protective asymptomatic" epitopes which: (i) are exclusively recognized by effector memory CD4(+) and CD8(+) T cells (TEM cells) from "naturally" protected seropositive asymptomatic individuals; and (ii) protect human leukocyte antigen (HLA) transgenic animal models of ocular and genital herpes. We review the role of animal models in herpes vaccine development and discuss their current status, challenges, and prospects.
Keywords: Asymptomatic; Clinical trials; Epitopes; Herpes simplex virus; Immunotherapeutic; Symptomatic; Vaccines.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures


References
-
- Samandary S, Kridane-Miledi H, Sandoval JS, Choudhury Z, Langa-Vives F DS, et al. Associations of HLA-A, HLA-B and HLA-C Alleles Frequency with Prevalence of Herpes Simplex Virus Infections and Diseases Across Global Populations: Implication for the Development of an Universal CD8+ T-Cell Epitope-Based Vaccine Human Immunoligt. 2014 Feb 5;5(5) - PMC - PubMed
-
- Dervillez X, Qureshi H, Chentoufi AA, Khan AA, Kritzer E, Yu DC, et al. Asymptomatic HLA-A*02:01-restricted epitopes from herpes simplex virus glycoprotein B preferentially recall polyfunctional CD8+ T cells from seropositive asymptomatic individuals and protect HLA transgenic mice against ocular herpes. J Immunol. 2013 Nov 15;191(10):5124–5138. - PMC - PubMed
-
- Zhang X, Dervillez X, Chentoufi AA, Badakhshan T, Bettahi I, BenMohamed L. Targeting the genital tract mucosa with a lipopeptide/recombinant adenovirus prime/boost vaccine induces potent and long-lasting CD8+ T cell immunity against herpes: importance of MyD88. J Immunol. 2012 Nov 1;189(9):4496–4509. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

