Pathogenesis of necrotizing enterocolitis: modeling the innate immune response
- PMID: 25447054
- PMCID: PMC4278236
- DOI: 10.1016/j.ajpath.2014.08.028
Pathogenesis of necrotizing enterocolitis: modeling the innate immune response
Abstract
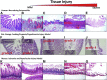
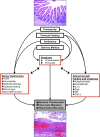
Necrotizing enterocolitis (NEC) is a major cause of morbidity and mortality in premature infants. The pathophysiology is likely secondary to innate immune responses to intestinal microbiota by the premature infant's intestinal tract, leading to inflammation and injury. This review provides an updated summary of the components of the innate immune system involved in NEC pathogenesis. In addition, we evaluate the animal models that have been used to study NEC with regard to the involvement of innate immune factors and histopathological changes as compared to those seen in infants with NEC. Finally, we discuss new approaches to studying NEC, including mathematical models of intestinal injury and the use of humanized mice.
Copyright © 2015 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Ballance W.A., Dahms B.B., Shenker N., Kliegman R.M. Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J Pediatr. 1990;117:S6–S13. - PubMed
-
- Lu P., Sodhi C.P., Jia H., Shaffiey S., Good M., Branca M.F., Hackam D.J. Animal models of gastrointestinal and liver diseases. Animal models of necrotizing enterocolitis: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol. 2014;306:G917–G928. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

