A 6-month study comparing efficacy, safety, and tolerability of the preservative-free fixed combination of tafluprost 0.0015% and timolol 0.5% versus each of its individual preservative-free components
- PMID: 25447269
- PMCID: PMC4271134
- DOI: 10.1007/s12325-014-0163-3
A 6-month study comparing efficacy, safety, and tolerability of the preservative-free fixed combination of tafluprost 0.0015% and timolol 0.5% versus each of its individual preservative-free components
Abstract
Introduction: The efficacy, safety and tolerability of the preservative-free (PF) fixed combination (FC) of tafluprost 0.0015% and timolol 0.5% (once daily) were compared to those of the individual components (PF tafluprost 0.0015% once daily and PF timolol 0.5% twice daily) in patients with open-angle glaucoma or ocular hypertension inadequately controlled on prior timolol or prostaglandin monotherapy for 6 months.
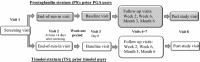
Methods: A stratified, double-masked, randomized, multicenter phase III study was conducted. A total of 189 prior timolol users were randomized within the timolol stratum (TS) to receive either FC (n = 95) or timolol 0.5% (TIM; n = 94). Furthermore, a total of 375 prior prostaglandin analog (PGA) users were randomized within the prostaglandin stratum (PS) to receive either FC (n = 188) or tafluprost 0.0015% (TAF; n = 187). To be eligible for participation in the study, the patients were required to have an intraocular pressure (IOP) of ≥22 mmHg when on timolol (TIM) or of ≥20 mmHg when on PGA in either treated eye at the screening and end-of-run-in visits. In addition to these, the study included visits at baseline, 2 and 6 weeks, 3 and 6 months and at a post-study visit. IOP was measured at 8 a.m., 10 a.m., 4 p.m., and 8 p.m.
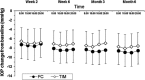
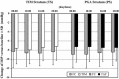
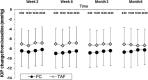
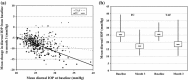
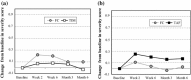
Results: In the TS, a significant reduction from baseline IOP was seen with FC and TIM throughout the study. Average diurnal IOP change from baseline at month 3 was -8.55 mmHg (32%) for FC and -7.35 mmHg (28%) for TIM. The model-based treatment difference (FC-TIM) was -0.885 mmHg [95% confidence interval (CI) -1.745 to -0.024; p = 0.044] demonstrating the superiority of FC over TIM. In the PS, a significant reduction in IOP was seen with both FC and TAF throughout the study. The average diurnal IOP change from baseline at month 3 was -8.61 mmHg (33%) for FC and -7.23 mmHg (28%) for TAF. The model-based treatment difference (FC-TAF) was -1.516 mmHg (95% CI -2.044 to -0.988; p < 0.001) demonstrating the superiority of FC over TAF. In the TS, related ocular adverse events (AEs) were more frequent for patients treated with FC compared to TIM (16.8% versus 6.4%), whereas related non-ocular AEs were more frequent with TIM compared to FC (2.1% versus 0.0%). In the PS, AEs were similarly distributed between FC and TAF. The frequency of conjunctival hyperemia of FC was low (6.4%).
Conclusion: The preservative-free fixed combination of tafluprost and timolol provided a substantial and significant IOP reduction in both strata. The IOP reduction was superior to both tafluprost 0.0015% and timolol 0.5% when given as monotherapies. Overall, the study treatments were safe and well tolerated.
Funding: Santen Oy, Tampere, Finland.
Trial registration: ClinicalTrials.gov NCT01292460.
Figures


References
-
- European Glaucoma Society . Terminology and Guidelines for Glaucoma. 4. Savona: PubliComm; 2014. p. 141.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

