High level of oxygen treatment causes cardiotoxicity with arrhythmias and redox modulation
- PMID: 25447406
- PMCID: PMC4426884
- DOI: 10.1016/j.taap.2014.10.019
High level of oxygen treatment causes cardiotoxicity with arrhythmias and redox modulation
Abstract
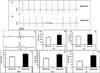
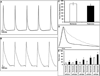
Hyperoxia exposure in mice leads to cardiac hypertrophy and voltage-gated potassium (Kv) channel remodeling. Because redox balance of pyridine nucleotides affects Kv function and hyperoxia alters cellular redox potential, we hypothesized that hyperoxia exposure leads to cardiac ion channel disturbances and redox changes resulting in arrhythmias. In the present study, we investigated the electrical changes and redox abnormalities caused by 72h hyperoxia treatment in mice. Cardiac repolarization changes were assessed by acquiring electrocardiogram (ECG) and cardiac action potentials (AP). Biochemical assays were employed to identify the pyridine nucleotide changes, Kv1.5 expression and myocardial injury. Hyperoxia treatment caused marked bradycardia, arrhythmia and significantly prolonged (ms) the, RR (186.2 ± 10.7 vs. 146.4 ± 6.2), PR (46.8 ± 3.1 vs. 39.3 ± 1.6), QRS (10.8 ± 0.6 vs. 8.5 ± 0.2), QTc (57.1 ± 3.5 vs. 40 ± 1.4) and JT (13.4 ± 2.1 vs. 7.0 ± 0.5) intervals, when compared with normoxia group. Hyperoxia treatment also induced significant increase in cardiac action potential duration (APD) (ex-APD90; 73.8 ± 9.5 vs. 50.9 ± 3.1 ms) and elevated levels of serum markers of myocardial injury; cardiac troponin I (TnI) and lactate dehydrogenase (LDH). Hyperoxia exposure altered cardiac levels of mRNA/protein expression of; Kv1.5, Kvβ subunits and SiRT1, and increased ratios of reduced pyridine nucleotides (NADH/NAD & NADPH/NADP). Inhibition of SiRT1 in H9C2 cells using Splitomicin resulted in decreased SiRT1 and Kv1.5 expression, suggesting that SiRT1 may mediate Kv1.5 downregulation. In conclusion, the cardiotoxic effects of hyperoxia exposure involve ion channel disturbances and redox changes resulting in arrhythmias.
Keywords: ECG; Heart; Hyperoxia; Kvβ; Potassium channel; Redox.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflict of interests. No disclosures.
Figures






References
-
- Amicarelli F, Ragnelli AM, Aimola P, Bonfigli A, Colafarina S, Di Ilio C, Miranda M. Agedependent ultrastructural alterations and biochemical response of rat skeletal muscle after hypoxic or hyperoxic treatments. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1999;1453:105–114. - PubMed
-
- Barry DM, Xu H, Schuessler RB, Nerbonne JM. Functional Knockout of the Transient Outward Current, Long-QT Syndrome, and Cardiac Remodeling in Mice Expressing a Dominant-Negative Kv4 Subunit. Circulation research. 1998;83:560–567. - PubMed
-
- Berul CI, Aronovitz MJ, Wang PJ, Mendelsohn ME. In Vivo Cardiac Electrophysiology Studies in the Mouse. Circulation. 1996;94:2641–2648. - PubMed
-
- Bin-Jaliah I, Dallak M, Haffor AS. Effect of hyperoxia on the ultrastructural pathology of alveolar epithelium in relation to glutathione peroxidase, lactate dehydrogenase activities, and free radical production in rats, Rattus norvigicus. Ultrastruct Pathol. 2009;33:112–122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

