Kinetics of the early events of GPCR signalling
- PMID: 25447525
- PMCID: PMC4266533
- DOI: 10.1016/j.febslet.2014.10.043
Kinetics of the early events of GPCR signalling
Abstract
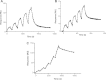
Neurotensin receptor type 1 (NTS1) is a G protein-coupled receptor (GPCR) that affects cellular responses by initiating a cascade of interactions through G proteins. The kinetic details for these interactions are not well-known. Here, NTS1-nanodisc-Gαs and Gαi1 interactions were studied. The binding affinities of Gαi1 and Gαs to NTS1 were directly measured by surface plasmon resonance (SPR) and determined to be 15±6 nM and 31±18 nM, respectively. This SPR configuration permits the kinetics of early events in signalling pathways to be explored and can be used to initiate descriptions of the GPCR interactome.
Keywords: Electron microscopy; G protein; G protein-coupled receptor; Nanodisc; Surface plasmon resonance.
Copyright © 2014 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Downes G.B., Gautam N. The G protein subunit gene families. Genomics. 1999;62:544–552. - PubMed
-
- Vincent J.P., Mazella J., Kitabgi P. Neurotensin and neurotensin receptors. Trends Pharmacol. Sci. 1999;20:302–309. - PubMed
-
- Harding P.J., Attrill H., Ross S., Koeppe J.R., Kapanidis A.N., Watts A. Neurotensin receptor type 1: Escherichia coli expression, purification, characterization and biophysical study. Biochem. Soc. Trans. 2007;35:760–763. - PubMed
-
- Pelaprat D. Interactions between neurotensin receptors and G proteins. Peptides. 2006;27:2476–2487. - PubMed
-
- Dobner P.R. Neurotensin and pain modulation. Peptides. 2006;27:2405–2414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

