Hepatitis C virus infection induces autocrine interferon signaling by human liver endothelial cells and release of exosomes, which inhibits viral replication
- PMID: 25447848
- PMCID: PMC4765499
- DOI: 10.1053/j.gastro.2014.10.040
Hepatitis C virus infection induces autocrine interferon signaling by human liver endothelial cells and release of exosomes, which inhibits viral replication
Abstract
Background & aims: Liver sinusoidal endothelial cells (LSECs) make up a large proportion of the nonparenchymal cells in the liver. LSECs are involved in induction of immune tolerance, but little is known about their functions during hepatitis C virus (HCV) infection.
Methods: Primary human LSECs (HLSECs) and immortalized liver endothelial cells (TMNK-1) were exposed to various forms of HCV, including full-length transmitted/founder virus, sucrose-purified Japanese fulminant hepatitis-1 (JFH-1), a virus encoding a luciferase reporter, and the HCV-specific pathogen-associated molecular pattern molecules. Cells were analyzed by confocal immunofluorescence, immunohistochemical, and polymerase chain reaction assays.
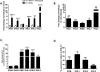
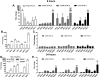
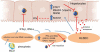
Results: HLSECs internalized HCV, independent of cell-cell contacts; HCV RNA was translated but not replicated. Through pattern recognition receptors (Toll-like receptor 7 and retinoic acid-inducible gene 1), HCV RNA induced consistent and broad transcription of multiple interferons (IFNs); supernatants from primary HLSECs transfected with HCV-specific pathogen-associated molecular pattern molecules increased induction of IFNs and IFN-stimulated genes in HLSECs. Recombinant type I and type III IFNs strongly up-regulated HLSEC transcription of IFN λ3 (IFNL3) and viperin (RSAD2), which inhibit replication of HCV. Compared with CD8(+) T cells, HLSECs suppressed HCV replication within Huh7.5.1 cells, also inducing IFN-stimulated genes in co-culture. Conditioned media from IFN-stimulated HLSECs induced expression of antiviral genes by uninfected primary human hepatocytes. Exosomes, derived from HLSECs after stimulation with either type I or type III IFNs, controlled HCV replication in a dose-dependent manner.
Conclusions: Cultured HLSECs produce factors that mediate immunity against HCV. HLSECs induce self-amplifying IFN-mediated responses and release of exosomes with antiviral activity.
Keywords: Cytokine; Immune Regulation; Innate Immunity; Toll-Like Receptor.
Copyright © 2015 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures




Comment in
-
Liver sinusoidal endothelial cells: an antiviral "defendothelium".Gastroenterology. 2015 Feb;148(2):288-91. doi: 10.1053/j.gastro.2014.12.010. Epub 2014 Dec 18. Gastroenterology. 2015. PMID: 25529809 No abstract available.
References
-
- Rosen HR. Clinical practice. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429–38. - PubMed
-
- Broering R, Lu M, Schlaak JF. Role of Toll-like receptors in liver health and disease. Clin Sci (Lond) 2011;121:415–26. - PubMed
-
- Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

