Epigenetic targets for novel therapies of lung diseases
- PMID: 25448041
- PMCID: PMC4323764
- DOI: 10.1016/j.pharmthera.2014.11.006
Epigenetic targets for novel therapies of lung diseases
Abstract
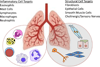
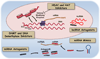
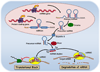
In spite of substantial advances in defining the immunobiology and function of structural cells in lung diseases there is still insufficient knowledge to develop fundamentally new classes of drugs to treat many lung diseases. For example, there is a compelling need for new therapeutic approaches to address severe persistent asthma that is insensitive to inhaled corticosteroids. Although the prevalence of steroid-resistant asthma is 5-10%, severe asthmatics require a disproportionate level of health care spending and constitute a majority of fatal asthma episodes. None of the established drug therapies including long-acting beta agonists or inhaled corticosteroids reverse established airway remodeling. Obstructive airways remodeling in patients with chronic obstructive pulmonary disease (COPD), restrictive remodeling in idiopathic pulmonary fibrosis (IPF) and occlusive vascular remodeling in pulmonary hypertension are similarly unresponsive to current drug therapy. Therefore, drugs are needed to achieve long-acting suppression and reversal of pathological airway and vascular remodeling. Novel drug classes are emerging from advances in epigenetics. Novel mechanisms are emerging by which cells adapt to environmental cues, which include changes in DNA methylation, histone modifications and regulation of transcription and translation by noncoding RNAs. In this review we will summarize current epigenetic approaches being applied to preclinical drug development addressing important therapeutic challenges in lung diseases. These challenges are being addressed by advances in lung delivery of oligonucleotides and small molecules that modify the histone code, DNA methylation patterns and miRNA function.
Keywords: Asthma; COPD; DNA methylation; Fibrosis; Histone code; Noncoding RNA.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
BSC, MB, CAS and WTG declare they have no conflicts of interest.
Figures

References
-
- Akbas F, Coskunpinar E, Aynaci E, Oltulu YM, Yildiz P. Analysis of serum micro-RNAs as potential biomarker in chronic obstructive pulmonary disease. Exp Lung Res. 2012;38:286–294. - PubMed
-
- Aleku M, Schulz P, Keil O, Santel A, Schaeper U, Dieckhoff B, Janke O, Endruschat J, Durieux B, Roder N, Loffler K, Lange C, Fechtner M, Mopert K, Fisch G, Dames S, Arnold W, Jochims K, Giese K, Wiedenmann B, Scholz A, Kaufmann J. Atu027, a liposomal small interfering RNA formulation targeting protein kinase N3, inhibits cancer progression. Cancer Res. 2008;68:9788–9798. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

