Proteins, platelets, and blood coagulation at biomaterial interfaces
- PMID: 25448722
- PMCID: PMC5001692
- DOI: 10.1016/j.colsurfb.2014.09.040
Proteins, platelets, and blood coagulation at biomaterial interfaces
Abstract
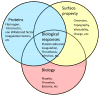
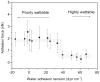
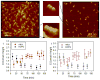
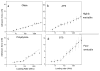
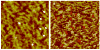
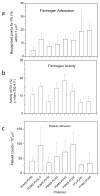
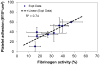
Blood coagulation and platelet adhesion remain major impediments to the use of biomaterials in implantable medical devices. There is still significant controversy and question in the field regarding the role that surfaces play in this process. This manuscript addresses this topic area and reports on state of the art in the field. Particular emphasis is placed on the subject of surface engineering and surface measurements that allow for control and observation of surface-mediated biological responses in blood and test solutions. Appropriate use of surface texturing and chemical patterning methodologies allow for reduction of both blood coagulation and platelet adhesion, and new methods of surface interrogation at high resolution allow for measurement of the relevant biological factors.
Keywords: Biological response; Biomaterials; Blood compatibility; Coagulation; Surfaces; Thrombosis.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures
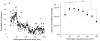





References
-
- Peppas NA, Langer R. New challanges in biomaterials. Science. 1994;263:1715–1720. - PubMed
-
- Hu WJ, Eaton JW, Ugarova TP, Tang L. Molecular basis of biomaterial-mediated foreign body reactions. Blood. 2001;98:1231–1238. - PubMed
-
- Anderson JM. Biological responses to materials. Annual Review of Materials Research. 2001;31:81–110.
-
- Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Seminars in Immunology. 2008;20:86–100. doi: http://dx.doi.org/10.1016/j.smim.2007.11.004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

