Cbl-family ubiquitin ligases and their recruitment of CIN85 are largely dispensable for epidermal growth factor receptor endocytosis
- PMID: 25449262
- PMCID: PMC4268055
- DOI: 10.1016/j.biocel.2014.10.019
Cbl-family ubiquitin ligases and their recruitment of CIN85 are largely dispensable for epidermal growth factor receptor endocytosis
Abstract
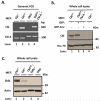
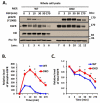
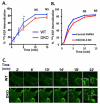
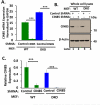
Members of the casitas B-lineage lymphoma (Cbl) family (Cbl, Cbl-b and Cbl-c) of ubiquitin ligases serve as negative regulators of receptor tyrosine kinases (RTKs). An essential role of Cbl-family protein-dependent ubiquitination for efficient ligand-induced lysosomal targeting and degradation is now well-accepted. However, a more proximal role of Cbl and Cbl-b as adapters for CIN85-endophilin recruitment to mediate ligand-induced initial internalization of RTKs is supported by some studies but refuted by others. Overexpression and/or incomplete depletion of Cbl proteins in these studies is likely to have contributed to this dichotomy. To address the role of endogenous Cbl and Cbl-b in the internalization step of RTK endocytic traffic, we established Cbl/Cbl-b double-knockout (DKO) mouse embryonic fibroblasts (MEFs) and demonstrated that these cells lack the expression of both Cbl-family members as well as endophilin A, while they express CIN85. We show that ligand-induced ubiquitination of EGFR, as a prototype RTK, was abolished in DKO MEFs, and EGFR degradation was delayed. These traits were reversed by ectopic human Cbl expression. EGFR endocytosis, assessed using the internalization of (125)I-labeled or fluorescent EGF, or of EGFR itself, was largely retained in Cbl/Cbl-b DKO compared to wild type MEFs. EGFR internalization was also largely intact in Cbl/Cbl-b depleted MCF-10A human mammary epithelial cell line. Inducible shRNA-mediated knockdown of CIN85 in wild type or Cbl/Cbl-b DKO MEFs had no impact on EGFR internalization. Our findings, establish that, at physiological expression levels, Cbl, Cbl-b and CIN85 are largely dispensable for EGFR internalization. Our results support the model that Cbl-CIN85-endophilin complex is not required for efficient internalization of EGFR, a prototype RTK.
Keywords: CIN85; Cbl; Epidermal growth factor receptor; Internalization; Mouse embryonic fibroblast.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures





References
-
- Bertelsen V, Sak MM, Breen K, Rodland MS, Johannessen LE, Traub LM, Stang E, Madshus IH. A chimeric pre-ubiquitinated EGF receptor is constitutively endocytosed in a clathrin-dependent, but kinase-independent manner. Traffic. 2011;12(4):507–20. - PubMed
-
- Dikic I. Mechanisms controlling EGF receptor endocytosis and degradation. Biochem Soc Trans. 2003;31(Pt 6):1178–81. - PubMed
-
- Dikic I. CIN85/CMS family of adaptor molecules. FEBS Lett. 2002;529(1):110–5. - PubMed
-
- Duan L, Raja SM, Chen G, Virmani S, Williams SH, Clubb RJ, Mukhopadhyay C, Rainey MA, Ying G, Dimri M, et al. Negative regulation of EGFR-Vav2 signaling axis by cbl ubiquitin ligase controls EGF receptor-mediated epithelial cell adherens junction dynamics and cell migration. J Biol Chem. 2011;286(1):620–33. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

