Cortical magnification plus cortical plasticity equals vision?
- PMID: 25449335
- PMCID: PMC4400204
- DOI: 10.1016/j.visres.2014.10.002
Cortical magnification plus cortical plasticity equals vision?
Abstract
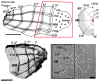
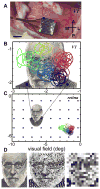
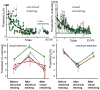
Most approaches to visual prostheses have focused on the retina, and for good reasons. The earlier that one introduces signals into the visual system, the more one can take advantage of its prodigious computational abilities. For methods that make use of microelectrodes to introduce electrical signals, however, the limited density and volume occupying nature of the electrodes place severe limits on the image resolution that can be provided to the brain. In this regard, non-retinal areas in general, and the primary visual cortex in particular, possess one large advantage: "magnification factor" (MF)-a value that represents the distance across a sheet of neurons that represents a given angle of the visual field. In the foveal representation of primate primary visual cortex, the MF is enormous-on the order of 15-20 mm/deg in monkeys and humans, whereas on the retina, the MF is limited by the optical design of the eye to around 0.3m m/deg. This means that, for an electrode array of a given density, a much higher-resolution image can be introduced into V1 than onto the retina (or any other visual structure). In addition to this tremendous advantage in resolution, visual cortex is plastic at many different levels ranging from a very local ability to learn to better detect electrical stimulation to higher levels of learning that permit human observers to adapt to radical changes to their visual inputs. We argue that the combination of the large magnification factor and the impressive ability of the cerebral cortex to learn to recognize arbitrary patterns, might outweigh the disadvantages of bypassing earlier processing stages and makes V1 a viable option for the restoration of vision.
Keywords: Magnification factor; Plasticity; Primary visual cortex; V1; Vision restoration; Visual prosthesis.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
References
-
- Auvray M, Hanneton S, O’Regan JK. Learning to perceive with a visuo-auditory substitution system: localisation and object recognition with ‘the vOICe’. Perception. 2007;36(3):416–430. - PubMed
-
- Bach-y-Rita P, Collins CC, Saunders FA, White B, Scadden L. Vision substitution by tactile image projection. Nature. 1969;221(5184):963–964. - PubMed
-
- Barlow H. What is the Computational Goal of the Neocortex? In: Koch C, Davis JL, editors. Large-Scale Neuronal Theories of the Brain. MIT Press; 1994. pp. 1–22.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

