Germline PRKACA amplification leads to Cushing syndrome caused by 3 adrenocortical pathologic phenotypes
- PMID: 25449630
- PMCID: PMC6309372
- DOI: 10.1016/j.humpath.2014.09.005
Germline PRKACA amplification leads to Cushing syndrome caused by 3 adrenocortical pathologic phenotypes
Abstract
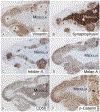
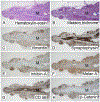
We describe the pathology of 5 patients with germline PRKACA copy number gain and Cushing syndrome: 4 males and 1 female, aged 2 to 43 years, including a mother and son. Imaging showed normal or slightly enlarged adrenal glands in 4 patients and a unilateral mass in the fifth. Biochemically, the patients had corticotropin-independent hypercortisolism. Four underwent bilateral adrenalectomy; unilateral adrenalectomy was performed in the patient with the adrenal mass. Pathologically, 3 patients, including the 1 with the tumor (adenoma), had primary pigmented nodular adrenocortical disease with extranodular cortical atrophy and mild intracapsular and extracapsular extension of cortical cells. The other 2 patients had cortical hyperplasia and prominent capsular and extracapsular micronodular cortical hyperplasia. Immunoperoxidase staining revealed differences for synaptophysin, inhibin-A, and Ki-67 (nuclei) in the atrophic cortices (patients 1, 2, and 3) and hyperplastic cortices (patients 4 and 5) and for Ki-67 (nuclei) and vimentin in the extracortical nodules in the 2 groups of patients. β-Catenin stained the cell membrane, cytoplasm, and nuclei of the adenoma. The patients were well at follow-up (1-23 years); 24-hour urinary cortisol excretion was elevated in the patient who had unilateral adrenalectomy.
Keywords: Adrenal; Cortical nodules; Cushing syndrome; Genetics; PRKACA.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest: The authors have no conflicts of interest or funding to disclose
Figures


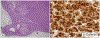
References
-
- Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations (pituitary basophilism). Bull Johns Hopkins Hosp. 1932;50:137–95.
-
- Shenoy BV, Carpenter PC, Carney JA. Bilateral primary pigmented nodular adrenocortical disease: rare cause of the Cushing syndrome. Am J Surg Pathol. 1984;8:335–44. - PubMed
-
- Carney JA, Libe R, Bertherat J, Young WF. Primary pigmented nodular adrenocortical disease: the original 4 cases revisited after 30 years for follow-up, new investigations, and molecular genetic findings. Am J Surg Pathol. 2014;38:1266–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

