Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis
- PMID: 25449861
- PMCID: PMC4514767
- DOI: 10.1002/da.22329
Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis
Abstract
Background: Randomized controlled trials have demonstrated that antidepressants are efficacious in the treatment of anxiety disorders in youth. However, there are no recent, systematic analyses of the efficacy, safety, or tolerability of these medications in pediatric anxiety disorders.
Methods: A systematic review and meta-analysis of prospective, randomized, parallel-group, controlled trials of selective serotonin reuptake inhibitors (SSRIs) and selective serotonin-norepinephrine reuptake inhibitors (SSNRIs) in pediatric patients with non-obsessive compulsive disorder (OCD) anxiety disorders was undertaken using a search of PubMed/Medline (1966-2014). The meta-analysis utilized random-effects models to evaluate change in the Pediatric Anxiety Rating Scale or similar anxiety scale, suicidality, and adverse events. Additionally, pharmacologic variables were explored with regard to effect size, although no correction for multiple comparisons was made with regard to these relationships.
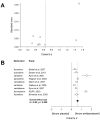
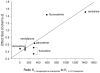
Results: Nine trials involving 1,673 patients and six medications were included. All SSRI/SSNRIs evaluated demonstrated efficacy, and the meta-analytic estimate of effect was of moderate magnitude (Cohen's d = 0.62, confidence interval [CI]: 0.34-0.89, P = .009) and there was evidence of modest heterogeneity (I(2) = 0.29, P = .103). Activation trended toward being more likely with antidepressant treatment (OR: 1.86, CI: 0.98-3.53, P = .054), but no increased risk was observed for nausea/abdominal symptoms (P = .262), discontinuation as a result of an adverse event (P = .132), or suicidality (OR: 1.3, CI: 0.53-3.2, P = .514). Finally, the effect size correlated with the serotonergic specificity of the agent (R = .79, P = .021).
Conclusions: Data for nine SSRI/SSNRIs suggest superiority of antidepressants relative to placebo for the treatment of pediatric anxiety disorders with a moderate effect size.
Keywords: GAD; antidepressants; anxiety; anxiety disorders; child/adolescent; generalized anxiety disorder; pharmacotherapy; separation anxiety.
© 2014 Wiley Periodicals, Inc.
Figures
Similar articles
-
Pharmacotherapy for anxiety and comorbid alcohol use disorders.Cochrane Database Syst Rev. 2015 Jan 20;1(1):CD007505. doi: 10.1002/14651858.CD007505.pub2. Cochrane Database Syst Rev. 2015. PMID: 25601826 Free PMC article.
-
New generation antidepressants for depression in children and adolescents: a network meta-analysis.Cochrane Database Syst Rev. 2021 May 24;5(5):CD013674. doi: 10.1002/14651858.CD013674.pub2. Cochrane Database Syst Rev. 2021. PMID: 34029378 Free PMC article.
-
Selective serotonin reuptake inhibitors, and serotonin and norepinephrine reuptake inhibitors for anxiety, obsessive-compulsive, and stress disorders: A 3-level network meta-analysis.PLoS Med. 2021 Jun 10;18(6):e1003664. doi: 10.1371/journal.pmed.1003664. eCollection 2021 Jun. PLoS Med. 2021. PMID: 34111122 Free PMC article.
-
Pharmacological treatments in panic disorder in adults: a network meta-analysis.Cochrane Database Syst Rev. 2023 Nov 28;11(11):CD012729. doi: 10.1002/14651858.CD012729.pub3. Cochrane Database Syst Rev. 2023. PMID: 38014714 Free PMC article.
-
Systematic Review and Meta-Analysis of Individual Participant Data: Randomized, Placebo-Controlled Trials of Selective Serotonin Reuptake Inhibitors for Pediatric Obsessive-Compulsive Disorder.J Am Acad Child Adolesc Psychiatry. 2025 Jul;64(7):775-785. doi: 10.1016/j.jaac.2025.01.001. Epub 2025 Jan 10. J Am Acad Child Adolesc Psychiatry. 2025. PMID: 39799995
Cited by
-
Escitalopram and Sertraline Population Pharmacokinetic Analysis in Pediatric Patients.Clin Pharmacokinet. 2023 Nov;62(11):1621-1637. doi: 10.1007/s40262-023-01294-8. Epub 2023 Sep 27. Clin Pharmacokinet. 2023. PMID: 37755681 Free PMC article.
-
Pharmacological treatment of anxiety disorders in children and adolescents: a review for practitioners.Transl Pediatr. 2018 Jan;7(1):23-35. doi: 10.21037/tp.2017.08.05. Transl Pediatr. 2018. PMID: 29441280 Free PMC article. Review.
-
Efficacy and Safety of Selective Serotonin Reuptake Inhibitors, Serotonin-Norepinephrine Reuptake Inhibitors, and Placebo for Common Psychiatric Disorders Among Children and Adolescents: A Systematic Review and Meta-analysis.JAMA Psychiatry. 2017 Oct 1;74(10):1011-1020. doi: 10.1001/jamapsychiatry.2017.2432. JAMA Psychiatry. 2017. PMID: 28854296 Free PMC article.
-
Neural Markers of Treatment Response in Pediatric Anxiety and PTSD.Curr Top Behav Neurosci. 2024 Dec 14:10.1007/7854_2024_547. doi: 10.1007/7854_2024_547. Online ahead of print. Curr Top Behav Neurosci. 2024. PMID: 39673034
-
Abnormalities of serotonergic neurotransmission in animal models of SUDEP.Epilepsy Behav. 2017 Jun;71(Pt B):174-180. doi: 10.1016/j.yebeh.2015.06.008. Epub 2015 Aug 10. Epilepsy Behav. 2017. PMID: 26272185 Free PMC article. Review.
References
-
- Beesdo K, Pine DS, Lieb R, Wittchen HU. Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Arch Gen Psychiatry. 2010;67(1):47–57. - PubMed
-
- Birmaher B, Axelson DA, Monk K, Kalas C, Clark DB, Ehmann M, Bridge J, Heo J, Brent DA. Fluoxetine for the treatment of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2003;42(4):415–423. - PubMed
-
- Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, Ren L, Brent DA. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;(297):1683–1696. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

