Colocalization of outflow segmentation and pores along the inner wall of Schlemm's canal
- PMID: 25450060
- PMCID: PMC4305530
- DOI: 10.1016/j.exer.2014.11.008
Colocalization of outflow segmentation and pores along the inner wall of Schlemm's canal
Abstract
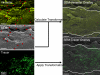
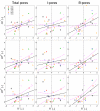
All aqueous humor draining through the conventional outflow pathway must cross the endothelium of Schlemm's canal (SC), likely by passing through micron-sized transendothelial pores. SC pores are non-uniformly distributed along the inner wall endothelium, but it is unclear how the distribution of pores relates to the non-uniform or segmental distribution of aqueous humor outflow through the trabecular meshwork. It is hypothesized that regions in the juxtacanalicular tissue (JCT) with higher local outflow should coincide with regions of greater inner wall pore density compared to JCT regions with lower outflow. Three pairs of non-glaucomatous human donor eyes were perfused at 8 mmHg with fluorescent tracer nanospheres to decorate local patterns of outflow segmentation through the JCT. The inner wall was stained for CD31 and/or vimentin and imaged en face using confocal and scanning electron microscopy (SEM). Confocal and SEM images were spatially registered to examine the spatial relationship between inner wall pore density and tracer intensity in the underlying JCT. For each eye, tracer intensity, pore density (n) and pore diameter (D) (for both transcellular "I" and paracellular "B" pores) were measured in 4-7 regions of interest (ROIs; 50 × 150 μm each). Analysis of covariance was used to examine the relationship between tracer intensity and pore density, as well as the relationship between tracer intensity and three pore metrics (nD, nD(2) and nD(3)) that represent the local hydraulic conductivity of the outflow pathway as predicted by various hydrodynamic models. Tracer intensity in the JCT correlated positively with local pore density when considering total pores (p = 0.044) and paracellular B pores on their own (p = 0.016), but not transcellular I-pores on their own (p = 0.54). Local hydraulic conductivity as predicted by the three hydrodynamic models all showed a significant positive correlation with tracer intensity when considering total pores and B-pores (p < 0.0015 and p < 10(-4)) but not I-pores (p > 0.38). These data suggest that aqueous humor passes through micron-sized pores in the inner wall endothelium of SC. Paracellular B-pores appear to have a dominant contribution towards transendothelial filtration across the inner wall relative to transcellular I-pores. Impaired pore formation, as previously described in glaucomatous SC cells, may thereby contribute to greater outflow heterogeneity, outflow obstruction, and IOP elevation in glaucoma.
Keywords: Endothelium; Human eyes; Inner wall; Juxtacanalicular tissue; Perfusion; Pores; Schlemm's canal; Segmentation.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures




References
-
- Allingham RR, de Kater AW, Ethier CR, Anderson PJ, Hertzmark E, Epstein DL. The relationship between pore density and outflow facility in human eyes. Investigative Ophthalmology & Visual Science. 1992;33:1661–1669. - PubMed
-
- Barton MD, Trembly BS. Experimental Eye Research. Experimental Eye Research. 2013;115:216–223. doi:10.1016/j.exer.2013.07.029. - DOI - PubMed
-
- Bill A, Svedbergh B. Scanning electron microscopic studies of the trabecular meshwork and the canal of Schlemm. Acta Ophthalmol (Copenh) 1972;50:295–320. - PubMed
-
- Braakman ST, Pedrigi RM, Read AT, Smith JAE, Stamer WD, Ethier CR, Overby DR. Experimental Eye Research. Experimental Eye Research. 2014;127:224–235. doi:10.1016/j.exer.2014.08.003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

