Redox regulation of mitophagy in the lung during murine Staphylococcus aureus sepsis
- PMID: 25450328
- PMCID: PMC4284964
- DOI: 10.1016/j.freeradbiomed.2014.10.582
Redox regulation of mitophagy in the lung during murine Staphylococcus aureus sepsis
Abstract
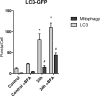
Oxidative mitochondrial damage is closely linked to inflammation and cell death, but low levels of reactive oxygen and nitrogen species serve as signals that involve mitochondrial repair and resolution of inflammation. More specifically, cytoprotection relies on the elimination of damaged mitochondria by selective autophagy (mitophagy) during mitochondrial quality control. This aim of this study was to identify and localize mitophagy in the mouse lung as a potentially upregulatable redox response to Staphylococcus aureus sepsis. Fibrin clots loaded with S. aureus (1×10(7) CFU) were implanted abdominally into anesthetized C57BL/6 and B6.129X1-Nfe2l2tm1Ywk/J (Nrf2(-/-)) mice. At the time of implantation, mice were given vancomycin (6mg/kg) and fluid resuscitation. Mouse lungs were harvested at 0, 6, 24, and 48h for bronchoalveolar lavage (BAL), Western blot analysis, and qRT-PCR. To localize mitochondria with autophagy protein LC3, we used lung immunofluorescence staining in LC3-GFP transgenic mice. In C57BL/6 mice, sepsis-induced pulmonary inflammation was detected by significant increases in mRNA for the inflammatory markers IL-1β and TNF-α at 6 and 24h, respectively. BAL cell count and protein also increased. Sepsis suppressed lung Beclin-1 protein, but not mRNA, suggesting activation of canonical autophagy. Notably sepsis also increased the LC3-II autophagosome marker, as well as the lung׳s noncanonical autophagy pathway as evidenced by loss of p62, a redox-regulated scaffolding protein of the autophagosome. In LC3-GFP mouse lungs, immunofluorescence staining showed colocalization of LC3-II to mitochondria, mainly in type 2 epithelium and alveolar macrophages. In contrast, marked accumulation of p62, as well as attenuation of LC3-II in Nrf2-knockout mice supported an overall decrease in autophagic turnover. The downregulation of canonical autophagy during sepsis may contribute to lung inflammation, whereas the switch to noncanonical autophagy selectively removes damaged mitochondria and accompanies tissue repair and cell survival. Furthermore, mitophagy in the alveolar region appears to depend on activation of Nrf2. Thus, efforts to promote mitophagy may be a useful therapeutic adjunct for acute lung injury in sepsis.
Keywords: Acute lung injury; Autophagy; Inflammation; LC3; Mitochondria; Mitophagy; Nrf2; Oxidative stress; Sepsis; p62.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures








References
-
- Annane D, Aegerter P, Jars-Guincestre MC, Guidet B. Current Epidemiology of Septic Shock. American Thoracic Society; 2012. http://dx.doi.org/10.1164/rccm.2201087. - DOI - PubMed
-
- Hotchkiss RS, Karl IE. The Pathophysiology and Treatment of Sepsis. N Engl J Med. 2003;348:138–150. - PubMed
-
- Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. British Journal of Anaesthesia. 2011;107:57–64. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

